2,3-dihydro-2-phenyl-1,5-benzothiazepin-4(5H)-one | 29476-22-0
中文名称
——
中文别名
——
英文名称
2,3-dihydro-2-phenyl-1,5-benzothiazepin-4(5H)-one
英文别名
2-phenyl-2,3-dihydrobenzo[b][1,4]thiazepin-4(5H)-one;2-Phenyl-2,3-dihydro-1,5-benzothiazepin-4(5H)-one;2-phenyl-3,5-dihydro-2H-1,5-benzothiazepin-4-one
CAS
29476-22-0
化学式
C15H13NOS
mdl
MFCD00158619
分子量
255.34
InChiKey
XIJZRXDBTDOULF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:18
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.133
-
拓扑面积:54.4
-
氢给体数:1
-
氢受体数:2
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : 2,3-DIHYDRO-2-PHENYL-1,5-BENZOTHIAZEPIN-
4(5H)-ONE
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 29476-22-0
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
This substance is not classified as dangerous according to Directive 67/548/EEC.
Label elements
This substance is not classified as dangerous according to Directive 67/548/EEC.
Other hazards - none
SECTION 3: Composition/information on ingredients
Substances
Formula : C15H13NOS
Molecular Weight : 255,34 g/mol
CAS-No. : 29476-22-0
No components need to be disclosed according to the applicable regulations.
SECTION 4: First aid measures
Description of first aid measures
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration.
In case of skin contact
Wash off with soap and plenty of water.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
no data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx), Sulphur oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Avoid dust formation. Avoid breathing vapours, mist or gas.
For personal protection see section 8.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Sweep up and shovel. Keep in suitable, closed containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Provide appropriate exhaust ventilation at places where dust is formed.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end use(s)
Apart from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
General industrial hygiene practice.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Choose body protection in relation to its type, to the concentration and amount of dangerous
substances, and to the specific work-place., The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection is not required. Where protection from nuisance levels of dusts are desired,
use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evapouration rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
SECTION 10: Stability and reactivity
Reactivity
no data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitisation
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Additional Information
RTECS: Not available
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
SECTION 12: Ecological information
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
Other adverse effects
no data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15: Regulatory information
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
For this product a chemical safety assessment was not carried out
SECTION 16: Other information
Further information
Copyright 2014 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,5-苯并硫氮杂卓-4(5H)-酮,5-乙酰基-2,3-二氢-2-苯基- 5-Acetyl-2,3-dihydro-2-phenyl-1,5-benzothiazepin-4(5H)-on 104505-67-1 C17H15NO2S 297.378 —— 1-oxo-2-phenyl-1,2,3,5-tetrahydro-1λ4-benzo[b][1,4]thiazepin-4-one 73859-98-0 C15H13NO2S 271.34 —— 2,3-Dihydro-2-phenyl-5-propionyl-1,5-benzothiazepin-4(5H)-on 109532-92-5 C18H17NO2S 311.404 —— 2,3-Dihydro-2-phenyl-5-phenacetyl-1,5-benzothiazepin-4(5H)-on 109532-93-6 C23H19NO2S 373.475 1,5-苯并硫氮杂卓-4(5H)-酮,5-苯甲酰-2,3-二氢-2-苯基- 5-Benzoyl-2,3-dihydro-2-phenyl-1,5-benzothiazepin-4(5H)-on 109532-94-7 C22H17NO2S 359.448 —— 3-(2-Nitrophenyl)sulfanyl-3-phenylpropanoic acid 98186-15-3 C15H13NO4S 303.339 —— 3-(2-aminophenylthio)-1-(2,5-dimethyl-1H-pyrrol-1-yl)-3-phenylpropan-1-one 1285679-02-8 C21H22N2OS 350.484 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5-methyl-2-phenyl-2,3-dihydrobenzo[b][1,4]thiazepin-4(5H)-one 97038-01-2 C16H15NOS 269.367 —— 2,3,4,5-Tetrahydro-2-phenyl-1,5-benzothiazepin 6012-71-1 C15H15NS 241.357 1,5-苯并硫氮杂卓-4(5H)-酮,5-乙酰基-2,3-二氢-2-苯基- 5-Acetyl-2,3-dihydro-2-phenyl-1,5-benzothiazepin-4(5H)-on 104505-67-1 C17H15NO2S 297.378 —— 1-oxo-2-phenyl-1,2,3,5-tetrahydro-1λ4-benzo[b][1,4]thiazepin-4-one 73859-98-0 C15H13NO2S 271.34 —— 2,3-Dihydro-2-phenyl-5-propionyl-1,5-benzothiazepin-4(5H)-on 109532-92-5 C18H17NO2S 311.404 —— 3-Phenyl-3-<2-(propionylamino)phenylthio>propionsaeure-ethylester 109532-99-2 C20H23NO3S 357.474 —— 5-(3-dimethylamino-propyl)-2-phenyl-2,3-dihydro-5H-benzo[b][1,4]thiazepin-4-one 95554-73-7 C20H24N2OS 340.489 —— 3-(4-oxo-2-phenyl-3,4-dihydro-2H-benzo[b][1,4]thiazepin-5-yl)-propionic acid 37012-92-3 C18H17NO3S 327.404 —— 3-<2-(Acetylamino)phenylthio>-3-phenylpropionsaeure-ethylester 109532-96-9 C19H21NO3S 343.447 硫西新 5-(2-(dimethylamino)ethyl)-2-phenyl-2,3-dihydrobenzo[b][1,4]thiazepin-4(5H)-one 5845-26-1 C19H22N2OS 326.462 —— (R)-5-(2-(dimethylamino)ethyl)-2-phenyl-2,3-dihydrobenzo[b][1,4]thiazepin-4(5H)-one —— C19H22N2OS 326.462 —— (4-Oxo-2-phenyl-3,4-dihydro-2H-benzo[b][1,4]thiazepin-5-yl)-acetic acid 89813-73-0 C17H15NO3S 313.377 —— 2,3-dihydro-2-phenyl-1,5-benzothiazepin-4(5H)-thione 29476-14-0 C15H13NS2 271.407 —— 2,3-dihydro-2-phenyl-1,5-benzothiazepin-4(5H)-one 1,1-dioxide 29476-17-3 C15H13NO3S 287.339 —— 2,3-Dihydro-2-phenyl-5-phenacetyl-1,5-benzothiazepin-4(5H)-on 109532-93-6 C23H19NO2S 373.475 —— 5-Benzyl-2-phenyl-2,3,4,5-tetrahydro-1,5-benzothiazepin-4-one 847486-38-8 C22H19NOS 345.465 —— 2-(4-Oxo-2-phenyl-2,3-dihydro-1,5-benzothiazepin-5-yl)propanoic acid 77262-05-6 C18H17NO3S 327.404 —— 5-[(2-Methylphenyl)methyl]-2-phenyl-2,3-dihydro-1,5-benzothiazepin-4-one 1428258-31-4 C23H21NOS 359.492 —— 5-(4-methoxybenzyl)-2-phenyl-2,3-dihydrobenzo[b][1,4]thiazepin-4(5H)-one 1428258-32-5 C23H21NO2S 375.491 —— 5-methyl-2-phenyl-2,3-dihydrobenzo[b][1,4]thiazepin-4(5H)-one 1,1-dioxide 145603-20-9 C16H15NO3S 301.366 —— 5-[(2-Bromophenyl)methyl]-2-phenyl-2,3-dihydro-1,5-benzothiazepin-4-one 1428258-30-3 C22H18BrNOS 424.361 —— 5-[2-(3-Chlorophenyl)-2-oxoethyl]-2-phenyl-2,3-dihydro-1,5-benzothiazepin-4-one 1428258-35-8 C23H18ClNO2S 407.92 —— 5-(2-fluorobenzyl)-2-phenyl-2,3-dihydrobenzo[b][1,4]thiazepin-4(5H)-one 847486-42-4 C22H18FNOS 363.455 —— 5-[(2-Chlorophenyl)methyl]-2-phenyl-2,3-dihydro-1,5-benzothiazepin-4-one 1428258-29-0 C22H18ClNOS 379.91 —— 3-[(4-Oxo-2-phenyl-2,3-dihydro-1,5-benzothiazepin-5-yl)methyl]benzoic acid 1428258-33-6 C23H19NO3S 389.475 —— Methyl 3-[(4-oxo-2-phenyl-2,3-dihydro-1,5-benzothiazepin-5-yl)methyl]benzoate 1428258-34-7 C24H21NO3S 403.502 1,5-苯并硫氮杂卓-4(5H)-酮,5-苯甲酰-2,3-二氢-2-苯基- 5-Benzoyl-2,3-dihydro-2-phenyl-1,5-benzothiazepin-4(5H)-on 109532-94-7 C22H17NO2S 359.448 —— 5-[(2-Nitrophenyl)methyl]-2-phenyl-2,3-dihydro-1,5-benzothiazepin-4-one 1414861-66-7 C22H18N2O3S 390.463 —— 3-(2-Aminophenylthio)-3-phenylpropionsaeure-ethylester 109532-97-0 C17H19NO2S 301.409 —— 5-methyl-2,3-dihydro-1,5-benzothiazepin-3(5H)-one 75620-24-5 C10H11NOS 193.269 —— 2-Benzyl-2H-1,4-benzothiazin-3(4H)-on 104505-74-0 C15H13NOS 255.34 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:2,3-dihydro-2-phenyl-1,5-benzothiazepin-4(5H)-one 在 盐酸 、 水 、 sodium hydride 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 7.5h, 生成 3-[(4-Oxo-2-phenyl-2,3-dihydro-1,5-benzothiazepin-5-yl)methyl]benzoic acid参考文献:名称:Design, synthesis and biological evaluation of benzothiazepinones (BTZs) as novel non-ATP competitive inhibitors of glycogen synthase kinase-3β (GSK-3β)摘要:Glycogen synthase kinase-3 beta (GSK-3 beta) plays a key role in type II diabetes and Alzheimer's diseases, to which non-ATP competitive inhibitors represent an effectively therapeutical approach due to their good specificity. Herein, a series of small molecules benzothiazepinones (BTZs) as novel non-ATP competitive inhibitors of GSK-3 beta have been designed and synthesized. The in vitro evaluation performed by luminescent assay showed most BTZ derivatives have inhibitory effects in micromolar scale. Among them compounds 61, 6t and 6v have the IC50 values of 25.0 mu M, 27.8 mu M and 23.0 mu M, respectively. Moreover 6v is devoid of any inhibitory activity in the assays to other thirteen protein kinases. Besides, SAR is analyzed and a hypothetical enzymatic binding mode is proposed by molecular docking study, which would be useful for new candidates design. (C) 2012 Elsevier Masson SAS. All rights reserved.DOI:10.1016/j.ejmech.2012.09.021
-
作为产物:描述:参考文献:名称:Levai, Albert; Puzicha, Gisbert, Synthetic Communications, 1985, vol. 15, # 7, p. 623 - 632摘要:DOI:
文献信息
-
Efficient Synthesis of 1,4-Thiazepanones and 1,4-Thiazepanes as 3D Fragments for Screening Libraries作者:Anil K. Pandey、Steven E. Kirberger、Jorden A. Johnson、Jennifer R. Kimbrough、Danika K. D. Partridge、William C. K. PomerantzDOI:10.1021/acs.orglett.0c01230日期:2020.5.15ring systems with highly 3D character and are currently underrepresented in fragment screening libraries. A nuclear magnetic resonance (NMR) fragment screen identified 1,4-acylthiazepanes as new BET (bromodomain and extraterminal domain) bromodomain ligands; however, an efficient and readily diversified synthesis for library development has not been reported. Here we report a one-pot synthesis using1,4-Thiazepanes 和 1,4-thiazepanones 代表具有高度 3D 特征的七元环系统,目前在片段筛选库中代表性不足。核磁共振 (NMR) 片段筛选将 1,4-酰基硫氮杂环鉴定为新的 BET(溴结构域和末端外结构域)溴结构域配体;然而,用于库开发的高效且易于多样化的合成尚未见报道。在这里,我们报告了使用 α,β-不饱和酯和 1,2-氨基硫醇形成 1,4-噻嗪酮作为具有高 3D 特征的 1,4-噻嗪酮的前体的一锅法合成。该反应在合理的时间(0.5-3 小时)内以良好的产率进行,并且可以耐受范围广泛的 α,β-不饱和酯。几个 1, 4-噻嗪类通过两步转化合成,并使用蛋白质观察 19F NMR 表征为新的 BET 溴结构域配体。这种合成应该可以方便地访问各种 3D 片段以筛选库。
-
Catalytic Enantioselective Synthesis of Protecting-Group-Free 1,5-Benzothiazepines作者:Sara Meninno、Chiara Volpe、Alessandra LattanziDOI:10.1002/chem.201700837日期:2017.4.32,3‐dihydro‐1,5‐benzothiazepinones, by an organocatalyzed sulfa‐Michael reaction of readily available α,β‐unsaturated N‐acyl pyrazoles with 2‐aminothiophenols followed by silica‐gel‐catalyzed lactamization, has been developed. The method proceeds under mild conditions at room temperature and it requires only 1 mol % catalyst loading, to give 2‐aryl/alkyl‐substituted 1,5‐benzothiazepines in generally
-
Formal [3 + 4] Annulation of α,β-Unsaturated Acyl Azoliums: Access to Enantioenriched N–<i>H</i>-Free 1,5-Benzothiazepines作者:Chao Fang、Tao Lu、Jindong Zhu、Kewen Sun、Ding DuDOI:10.1021/acs.orglett.7b01457日期:2017.7.7An unprecedented formal [3 + 4] annulation of α,β-unsaturated acyl azoliums with 2-aminobenzenethiols has been utilized to synthesize enantioenriched N–H-free 1,5-benzothiazepines, which are recognized as privileged structures in numerous biologically active scaffolds. This protocol offers a rapid and direct pathway to access the target compounds with high enantioselectivities and has been applied
-
Synthesis and Biological Activity ofN-2 Alkylamino Derivatives of 4,5-Dihydro-s-triazolo[3,4-d]-1,5-benzothiazepine作者:Valeria Ambrogi、Antonio Giampietri、Giuliano Grandolini、Luana Perioli、Maurizio Ricci、Lorenzo TuttobelloDOI:10.1002/ardp.19923250909日期:——The synthesis of a new series of N‐2 alkylamino derivatives of 4,5‐dihydro‐s‐triazolo[3,4‐d]‐1,5‐benzothiazepine has been accomplished starting from 2,3‐dihydro‐1,5‐benzothiazepin‐4(5H)ones and their 2‐methyl and 2‐aryl derivatives. ‐ All the compounds were tested in vitro for their antimicrobial activity, but none of them showed remarkable activity. ‐ The tricyclic compounds 7a‐j, 8a‐j, 9a‐j, 10a‐j
-
Oxazepines and Thiazepines, XXV: Chemical Transformations of 2,3-Dihydro-1,5-benzothiazepin-4(5H)-ones作者:Albert LévaiDOI:10.1002/ardp.19923251108日期:——2,3‐Dihydro‐1,5‐benzothiazepine‐4(5H)‐thiones 13‐22 were prepared by the reaction of the appropriate 2,3‐dihydro‐1,5‐benzothiazepin‐4(5H)‐ones with Lawesson's reagent. N‐Acyl (23‐25) and N‐alkyl (26‐28) derivatives have also been synthesized. Oxidation with 3‐chloroperoxybenzoic acid afforded sulfoxides 29‐32, and sulfones 33‐40 were obtained by using H2O2 as an oxidizing agent.
表征谱图
-
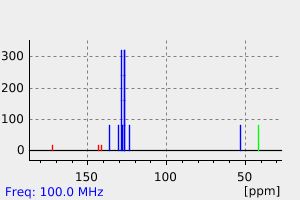
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐瑞索韦
马来酸地尔硫卓
贝匹斯汀
苯甲醇,-α--(1-氨基-2-丙烯基)-(9CI)
硫西新
盐酸地尔硫卓O-去乙酰化物
盐酸地尔硫卓
盐酸地尔硫卓
氯噻平
氟水杨基<邻羟苄基>醛
杂质L
尼克噻嗪
富马酸喹硫平
奎硫平去羟乙基杂质
奎硫平乙醚(富马酸)
奎硫平DBTO砜
地尔硫卓肾上腺素
地尔硫卓杂质8
地尔硫卓杂质5
地尔硫卓杂质4
地尔硫卓杂质
地尔硫卓EP杂质A
地尔硫卓-d6
地尔硫卓
喹硫平砜
喹硫平杂质E
喹硫平杂质DHCl
喹硫平杂质7
喹硫平杂质21
喹硫平杂质18
喹硫平亚砜
喹硫平二聚体
喹硫平EP杂质S盐
喹硫平-d8富马酸酯
喹硫平-D8半富马酸盐(哌嗪-D8)
喹硫平 N-氧化物
喹硫平
哌苯硫氮杂卓
哌嗪,3,3-二甲基-1-(1-甲基乙基)-(9CI)
去乙酰基地尔硫卓N-氧化物
去乙酰地尔硫卓
去乙酰-O-去甲基地尔硫卓
克仑硫卓
倍氯米松杂质D
二苯并[b,f]咪唑并[1,2-d][1,4]硫氮杂卓
二苯并[b,f][1,4]硫氮杂卓-11-胺
二苯并[b,f][1,4]硫氮杂卓-11-[10H]酮
二苯并(b,f)-1,2,4-三唑并(4,3-d)(1,4)硫氮杂卓-6-胺
rel-(2R,3R)-3-(乙酰氧基)-5-[2-(二甲基氨基)乙基]-2,3-二氢-8-甲氧基-2-(4-甲氧基苯基)-1,5-苯并噻嗪-4(5H)-酮
rel-(2R,3R)-2,3-二氢-3-羟基-2-(4-甲基苯基)-1,5-苯并噻唑啉-4(5H)-酮