N-(3,4-dimethylphenyl)-4-methylbenzenesulfonamide | 114097-27-7
中文名称
——
中文别名
——
英文名称
N-(3,4-dimethylphenyl)-4-methylbenzenesulfonamide
英文别名
——
CAS
114097-27-7
化学式
C15H17NO2S
mdl
MFCD01181378
分子量
275.371
InChiKey
NQUKYTXPMUKVOZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:144.5-145.0 °C
-
沸点:418.5±55.0 °C(Predicted)
-
密度:1.211±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:19
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:54.6
-
氢给体数:1
-
氢受体数:3
安全信息
-
储存条件:室温
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-(3,4-二甲基苯基)-N,4-二甲基-苯磺酰胺 4-(N-Methyl-p-toluolsulfonylamino)-o-xylol 408508-82-7 C16H19NO2S 289.398
反应信息
-
作为反应物:描述:参考文献:名称:作为取代过程中可能的中间体的添加剂化合物。第二部分摘要:DOI:10.1039/jr9550002376
-
作为产物:描述:对甲基苯磺酰异氰酸酯 、 4,5-dimethyl-2-(trimethylsilyl)phenyl triflate 在 cesium fluoride 作用下, 以 乙腈 为溶剂, 反应 14.0h, 以72%的产率得到N-(3,4-dimethylphenyl)-4-methylbenzenesulfonamide参考文献:名称:通过苯炔与异氰酸酯的反应无过渡金属合成芳族胺摘要:在不含过渡金属的条件下,苯并炔与异氰酸酯发生意外反应生成芳族胺。在原位制备过N开裂而形成的阴离子C键中的异氰酸酯,具有芳炔前体在中等至良好的产率反应,得到各种苯胺衍生物和耐受上各种取代基ø -甲硅烷基三氟甲磺酸的芳基和异氰酸酯。DOI:10.1016/j.tetlet.2018.01.022
文献信息
-
Accessing bridged bicyclic compounds or meta carbon-functionalized anilines from the dearomatization of anilines作者:Linfei Wang、Shuo-En Wang、Weibin Wang、Renhua FanDOI:10.1039/c3ra23224g日期:——The oxidative dearomatization of anilines was combined with a domino Michael addition, providing a series of nitrogen-containing bridged bicyclic compounds in moderate to excellent yields. The bridged bicyclic compound could be converted into the corresponding meta carbon-functionalized aniline derivative via a quinine-catalyzed tandem retro-oxa-Michael addition–aromatization reaction.
-
Iodine-mediated regioselective C2-amination of indoles and a concise total synthesis of (±)-folicanthine作者:Ying-Xiu Li、Hai-Xi Wang、Shaukat Ali、Xiao-Feng Xia、Yong-Min LiangDOI:10.1039/c2cc16637b日期:——Highly substituted 2-aminated indoles can be prepared in moderate to excellent yields by regioselective C2-amination of indoles promoted by iodine. As a key step in a concise synthesis of (+/-)-folicanthine, its core structure was easily obtained by one step cyclization-dimerization of substituted tryptophan in high yield on a gram scale.
-
A Catalytic Construction of Indoles via Formation of Ruthenium Vinylidene Species from <i>N</i> ‐Arylynamides作者:Masanori Tayu、Ryuta Watanabe、Satoshi Isogi、Nozomi SaitoDOI:10.1002/adsc.202001342日期:2021.2.16with a catalytic amount of TpRuCl(PPh3)2 resulted in the construction of indole scaffolds known as privileged structure motifs. This reaction involved a cascade of 1,2‐rearrangement and cyclization carrying out C−C bond formation via a ruthenium vinylidene intermediate, as revealed by a deuterium‐labeling experiments. Furthermore, the transformation of multi‐functionalized ynamide, derived from a practical
-
Synthesen in der Lumiflavinreihe作者:P. Hemmerich、S. Fallab、H. ErlenmeyerDOI:10.1002/hlca.19560390511日期:——A short way for the synthesis of lumiflavin and derivatives is developed, leading to the new structural analogues 2-thiolumiflavine and 2-lumiflavimine, which exhibit interesting oxido-reductive and metal chelating properties and may be riboflavin antirnetabolites.
-
Stereoselective Synthesis of Acyclic Tetrasubstituted Alkenes from Anilines by Dearomatization and Trimethylenemethane Cycloaddition作者:Lei Li、Qiuqin He、Renhua FanDOI:10.1021/acs.orglett.1c03974日期:2022.1.14A method for stereoselective construction of acyclic all-carbon tetrasubstituted alkenes through insertion of nitrile-substituted trimethylenemethane into the aryl C–N bond in anilines via an aromaticity destruction-reconstruction process is reported. The process involves dearomatization, azo-[3 + 2] TMM cycloaddition and aromatization-triggered rearrangement.
表征谱图
-
氢谱1HNMR
-
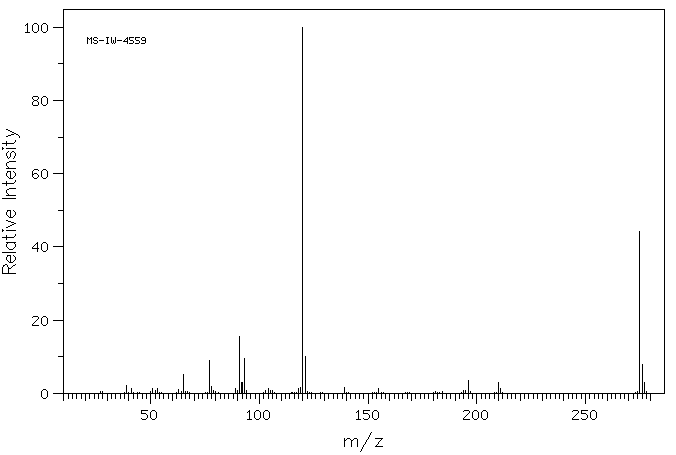
质谱MS
-
碳谱13CNMR
-
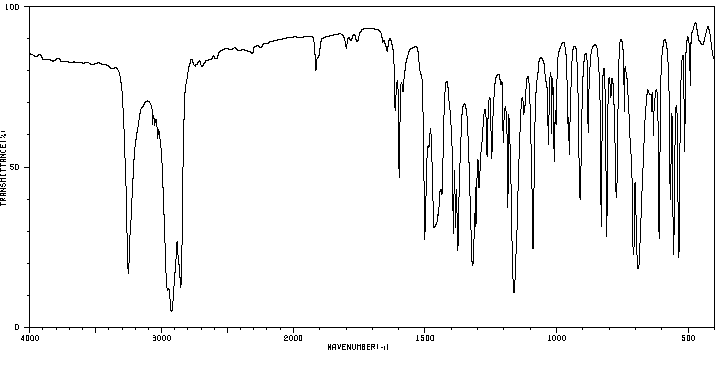
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫