N,N-bis(trimethylsilyl)-glycine trimethylsilyl ester | 5630-82-0
中文名称
——
中文别名
——
英文名称
N,N-bis(trimethylsilyl)-glycine trimethylsilyl ester
英文别名
N,N-bis(trimethylsilyl)glycine trimethylsilyl ester;N,N-Bis(trimethylsilyl)glycine trimethylsilylester;N,N-Bis(trimethylsilyl)glycin-trimethylsilylester;N,N,O-Trimethylsilylglycine;glycine TMS;N,N-Bis-(trimethylsilyl)-glycin-trimethylsilylester;Trimethylsilyl [bis(trimethylsilyl)amino]acetate;trimethylsilyl 2-[bis(trimethylsilyl)amino]acetate
CAS
5630-82-0
化学式
C11H29NO2Si3
mdl
——
分子量
291.613
InChiKey
YVBMCPFZLGEMJT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:108-109 °C(Press: 12 Torr)
-
密度:0.888±0.06 g/cm3(Predicted)
-
保留指数:1317;1315.88;1326.3
计算性质
-
辛醇/水分配系数(LogP):3.34
-
重原子数:17
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.91
-
拓扑面积:29.5
-
氢给体数:0
-
氢受体数:3
SDS
反应信息
-
作为反应物:参考文献:名称:合成肽6-硝基-2-氨基己酮摘要:DOI:10.1016/s0040-4039(01)86804-0
-
作为产物:参考文献:名称:用-双(三甲基甲硅烷基)氨基乙烯酮双(三甲基甲硅烷基)乙缩醛或其-甲基-三甲基甲硅烷基类似物合成α-氨基-β-羟基酸摘要:在三氟甲磺酸三甲基甲硅烷基酯的存在下,标题化合物与醛和酮的缩合以非对映异构体混合物的形式以相当高的收率提供了相应的α-氨基-β-羟基酸。DOI:10.1016/s0040-4039(00)83885-x
文献信息
-
29Si and13C NMR spectra of trimethylsilylated amino acids作者:Jan Schraml、Magdalena Kvíčalová、Iveta Schwarzová、Jan VelíšekDOI:10.1002/mrc.1260321005日期:1994.10The 29Si and 13C NMR chemical shifts are reported for trimethylsilyl derivatives of 25 amino acids, the majority of which occur naturally as protein constituents of foodstuffs. The 29Si chemical shifts in trimethylsilyl esters of amino acids roughly correlate linearly with the pK values of the parent amino acid. No such simple correlations were found for the shifts in the fully trimethylsilylated amino
-
Glycine Imine—The Elusive <i>α</i> ‐Imino Acid Intermediate in the Reductive Amination of Glyoxylic Acid作者:Viktor Paczelt、Raffael C. Wende、Peter R. Schreiner、André K. EckhardtDOI:10.1002/anie.202218548日期:2023.3.62-Iminoacetic acid or glycine imine is a proposed key intermediate in the reductive amination of glyoxylic acid. In contrast to well-known α-amino acids the compound class of α-imino acids is not much explored and not well-characterized. Glycine imine was prepared through photochemical denitrogenation of 2-azidoacetic acid, spectroscopically characterized, and its reactivity studied in aqueous solution
-
Schorr, Manfred; Schmitt, Wilfried, Phosphorus, Sulfur and Silicon and the Related Elements, 1992, vol. 68, # 1-4, p. 25 - 36作者:Schorr, Manfred、Schmitt, WilfriedDOI:——日期:——
-
Synthesis of (4)-4-(()-2-butenyl)-4,-dimethyl--threonine (MeBMT), the characteristic amino acid of cyclosporine作者:Ulrich Schmidt、Wolfgang SiegelDOI:10.1016/s0040-4039(00)96225-7日期:1987.1
-
Lipase-Catalyzed Enantiomeric Resolution of Ceramides作者:Mikio Bakke、Masahiro Takizawa、Takeshi Sugai、Hiromichi OhtaDOI:10.1021/jo980727u日期:1998.10.1Lipase-catalyzed enantiomeric kinetic resolution of ceramides related to C-16-sphinganine and C-18-sphingenine is described. Two hydroxy groups in readily available racemic N-stearoyl-erythro-C-16-sphinganine were acetylated, and several kinds of lipases were screened for the hydrolysis of this substrate. Among them, a Burkholderia cepacia lipase (SC lipase A, Sumitomo Chemical Co., Ltd.) showed the highest reactivity and enantioselectivity. The rate of hydrolysis and selectivity were greatly affected by some additives. Especially, the combined use of a detergent, Triton X-100, and the solid support, Florisil, for immobilization showed the highest enantioselectivity (E = ca. 170), although the reaction rate turned low. Introduction of a double bond into the substrate (N-stearoyl-erythro-C-18-sphingenine) also retarded the hydrolysis. By utilizing the preferential hydrolysis of the acetate on the primary hydroxy group, another advantageous feature of this enzyme-catalyzed reaction, the resulting product could directly be used as the glycosyl acceptor for cerebroside synthesis.
表征谱图
-
氢谱1HNMR
-
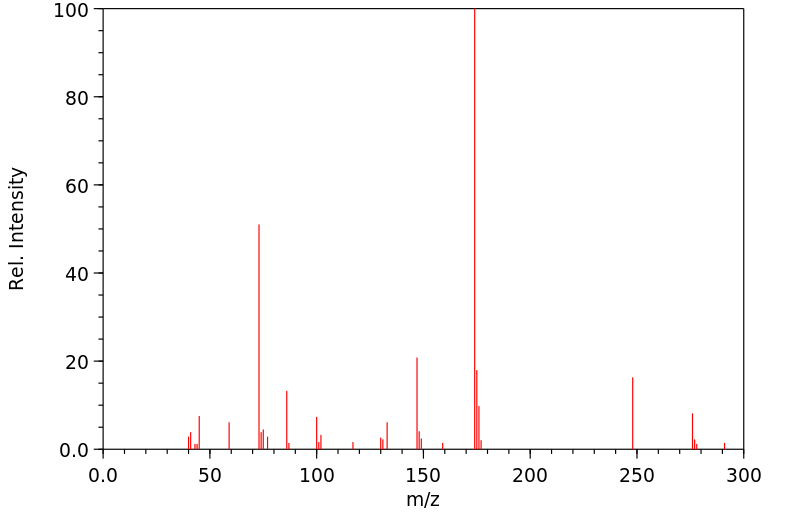
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸