5,6-dihydro-4H-benzo[3,4]cyclohepta[d][1,3]thiazol-2-amine | 21600-17-9
中文名称
——
中文别名
——
英文名称
5,6-dihydro-4H-benzo[3,4]cyclohepta[d][1,3]thiazol-2-amine
英文别名
5,6-dihydro-4H-benzo[6,7]cyclohepta[1,2-d]thiazol-2-amine;2-Amino-9,10-dihydro-8H-benzo<4.5>cycloheptathiazol;2-Amino-9.10-dehydro-8H-benzo<4.5>cycloheptathiazol;2-Amino-9.10-dehydro-8H-benzo[4.5]cyclohepta[d]thiazol;5-thia-3-azatricyclo[8.4.0.02,6]tetradeca-1(14),2(6),3,10,12-pentaen-4-amine
CAS
21600-17-9
化学式
C12H12N2S
mdl
——
分子量
216.307
InChiKey
NWKXBHRPLGONGL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:444.2±14.0 °C(Predicted)
-
密度:1.281±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:15
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:67.2
-
氢给体数:1
-
氢受体数:3
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-prop-2-ynoxy-N-(5-thia-3-azatricyclo[8.4.0.02,6]tetradeca-1(14),2(6),3,10,12-pentaen-4-yl)benzamide 1431658-53-5 C22H18N2O2S 374.463
反应信息
-
作为反应物:描述:5,6-dihydro-4H-benzo[3,4]cyclohepta[d][1,3]thiazol-2-amine 以 乙醇 为溶剂, 反应 6.0h, 生成 10-phenyl-6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-d]imidazo[2,1-b][1,3]thiazole参考文献:名称:Synthesis of phenylimidazo thiazolo benzocycloheptene derivatives as potential antiinflammatory agent-IV†摘要:DOI:10.1515/hc.2001.7.6.535
-
作为产物:参考文献:名称:Synthesis of phenylimidazo thiazolo benzocycloheptene derivatives as potential antiinflammatory agent-IV†摘要:DOI:10.1515/hc.2001.7.6.535
文献信息
-
Visible-Light-Driven Synthesis of 4-Alkyl/Aryl-2-Aminothiazoles Promoted by In Situ Generated Copper Photocatalyst作者:Wen-Long Lei、Tao Wang、Kai-Wen Feng、Li-Zhu Wu、Qiang LiuDOI:10.1021/acscatal.7b02818日期:2017.11.3Room-temperature synthesis of 4-alkyl/aryl-2-aminothiazoles from vinyl azides and ammonium thiocyanate was accomplished with the aid of copper salts and blue LED irradiation. Mechanism investigation indicates that in situ-formed Cu(NCS)2– plays dual important roles in the reaction: (1) as the photocatalyst to activate vinyl azides, (2) as the Lewis acid catalyst to promote ring opening of 2H-azirines
-
一种可见光驱动合成4-烷基或芳基-2-氨基噻唑的方法
-
Selective NPY (Y5) antagonists申请人:Marzabadi R. Mohammad公开号:US20080045524A1公开(公告)日:2008-02-21This invention is directed to triazine derivatives, bicyclic compounds and tricyclic compounds which are selective antagonists for a NPY (Y5) receptor. The invention provides a pharmaceutical composition comprising a therapeutically effective amount of a compound of the invention and a pharmaceutically acceptable carrier. This invention provides a pharmaceutical composition made by combining a therapeutically effective amount of a compound of this invention and a pharmaceutically acceptable carrier. This invention provides a process for making a pharmaceutical composition comprising combining a therapeutically effective amount of a compound of the invention and a pharmaceutically acceptable carrier. The invention further provides the use of a compound of the invention for the preparation of a pharmaceutical composition for treating an abnormality, wherein the abnormality is alleviated by decreasing the activity of a human Y5 receptor.
-
FUSED RING COMPOUNDS FOR INFLAMMATION AND IMMUNE-RELATED USES申请人:Che Quinglin公开号:US20080132513A1公开(公告)日:2008-06-05The invention relates to certain fused ring compounds, or pharmaceutically acceptable salts, solvates, clathrates, or prodrugs thereof, that are useful as immunosuppressive agents and for treating and preventing inflammatory conditions, allergic disorders, and immune disorders.本发明涉及某些融合环化合物,或其药学上可接受的盐,溶剂合物,笼合物或前药,它们可用作免疫抑制剂,用于治疗和预防炎症状况,过敏性疾病和免疫障碍。
-
Tricyclic thiazoles are a new class of angiogenesis inhibitors作者:Shridhar Bhat、Joong Sup Shim、Jun O. LiuDOI:10.1016/j.bmcl.2013.02.067日期:2013.5Tricyclic thiazoleamine derivatives that were identified as hits in a screen against human umbilical vein endothelial cell proliferation were subjected to a structure-activity relationship study. Two structurally superimposable scaffolds-4H-thiochromeno[4,3-d]thiazol-2-amine and 5,6-dihydro-4H-benzo[6,7]cyclo hepta[1,2-d] thiazol-2-amine derivatives-yielded low-micromolar inhibitors, and two among them 37 and 43 also exhibited antiangiogenic activity in an endothelial tube formation assay. Thus, 37 and 43 can serve as leads to develop a novel class of antiangiogenic agents. (C) 2013 Elsevier Ltd. All rights reserved.
表征谱图
-
氢谱1HNMR
-
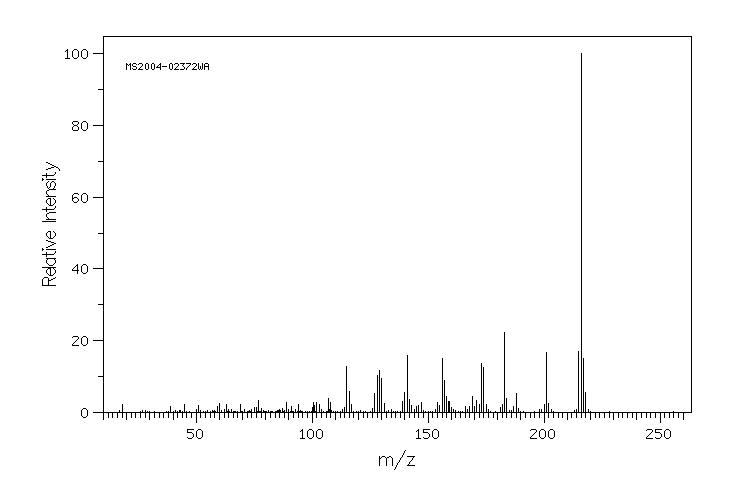
质谱MS
-
碳谱13CNMR
-
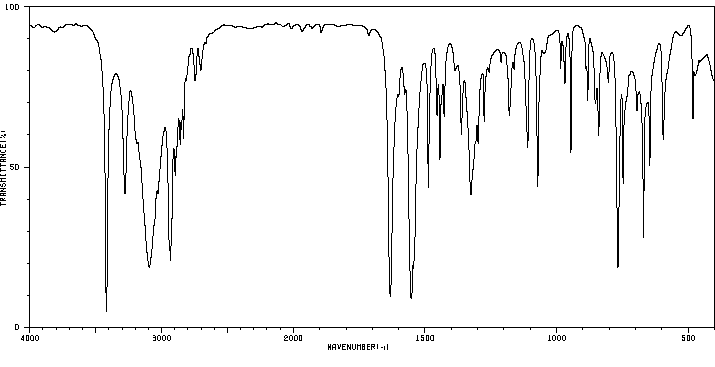
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-(+)-5,5'',6,6'',7,7'',8,8''-八氢-3,3''-二叔丁基-1,1''-二-2-萘酚,双钾盐
(S)-盐酸沙丁胺醇
(S)-溴烯醇内酯
(S)-7,7-双[(4S)-(苯基)恶唑-2-基)]-2,2,3,3-四氢-1,1-螺双茚满
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2-N-Fmoc-氨基甲基吡咯烷盐酸盐
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-7,7-双[(4S)-(苯基)恶唑-2-基)]-2,2,3,3-四氢-1,1-螺双茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(6,6)-苯基-C61己酸甲酯
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,5R)-3,3a,8,8a-四氢茚并[1,2-d]-1,2,3-氧杂噻唑-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aS,8aR)-2-(吡啶-2-基)-8,8a-二氢-3aH-茚并[1,2-d]恶唑
(3aS,3''aS,8aR,8''aR)-2,2''-环戊二烯双[3a,8a-二氢-8H-茚并[1,2-d]恶唑]
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(3-三苯基甲氨基甲基)吡啶
(3-[(E)-1-氰基-2-乙氧基-2-hydroxyethenyl]-1-氧代-1H-茚-2-甲酰胺)
(2′′-甲基氨基-1,1′′-联苯-2-基)甲烷磺酰基铝(II)二聚体
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,4S)-Fmoc-4-三氟甲基吡咯烷-2-羧酸
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环