5-叔-丁基焦酚 | 20481-17-8
中文名称
5-叔-丁基焦酚
中文别名
5-叔丁基焦棓酚
英文名称
5-tert-butylpyrogallol
英文别名
5-t-butylpyrogallol;3-hydroxy-5-tert-butyl-catechol;5-tert-butylbenzene-1,2,3-triol
CAS
20481-17-8
化学式
C10H14O3
mdl
MFCD00059613
分子量
182.219
InChiKey
HCNISNCKPIVZDX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:133-136°C
-
沸点:348.4±37.0 °C(Predicted)
-
密度:1.203±0.06 g/cm3(Predicted)
-
保留指数:1526
-
稳定性/保质期:
如果遵照规格使用和储存,则不会分解。避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:13
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:60.7
-
氢给体数:3
-
氢受体数:3
安全信息
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
海关编码:2907299090
-
储存条件:保持贮藏器密封,并将其放入一个紧密封装的容器中。应存放在阴凉、干燥的地方。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-叔丁基-2,6-二甲氧基苯酚 4-(tert-butyl)-2,6-dimethoxyphenol 6766-84-3 C12H18O3 210.273 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5-tert.-Butyl-pyrogallol-trimethylether 19589-29-8 C13H20O3 224.3
反应信息
-
作为反应物:描述:5-叔-丁基焦酚 在 tetrasulphur tetranitride 作用下, 以 甲苯 为溶剂, 反应 24.0h, 以5%的产率得到4-t-butylbenzo<1,2-c:3:4-c'>bis<1,2,5>thiadiazole参考文献:名称:有机化学中的氮化硫。18. 苯并[1,2-c:3,4-c':5,6-c″]tris-和苯并[1,2-c:3,4-c']bis[1, 2,5]噻二唑。获得苯六胺和 1,2,3,4-苯四胺的便捷途径摘要:通过四氮化四硫 (5) 与卤代儿茶酚 (6) 和间苯二酚 (7) 的反应,分别以中等产率制备苯并三- (3) 和苯并双 [1,2,5] 噻二唑 (4)。在浓盐酸和二恶烷的混合物中用锡粉还原 3 和 4 得到苯六胺 (1) 和 1,2,3,4-苯四胺 (2a) 及其 5-甲基衍生物 (2b),收率良好,如分别是稳定的三盐酸盐 (1·3HCl) 和二盐酸盐 (2·2HCl)。DOI:10.1246/bcsj.62.3127
-
作为产物:描述:参考文献:名称:Process for preparing pyrogallol摘要:白脲酚是通过以下步骤制备的:(A)将化学式为##STR1##的卤代化合物与化学式为ROM的碱金属烷氧化物反应,其中X代表Br或I,R.sup.1代表氢、长达10个碳原子的二级或三级烷基、2-5个碳原子的羧基或烷氧羰基,R.sup.2代表氢或1-4个碳原子的烷基,以替换每个X为OR;然后(B)脱烷基化每个OR和每个OR.sup.2,其中R.sup.2代表1-4个碳原子的烷基,并去除R.sup.1基团,如果R.sup.1不是氢的话。公开号:US04172960A1
文献信息
-
The constitution of the dimers of 4,6-di-t-butyl-3-hydroxy-o-benzoquinone and 5-t-butyl-3-hydroxy-o-benzoquinone作者:N. M. WaldronDOI:10.1039/j39680001914日期:——Evidence is presented which shows that the yellow and white dimers of 4,6-di-t-butyl-3-hydroxy-o-benzoquinone are structures 3,4a,6,8-tetra-t-butyl-4a, 10a-dihydro-9,10a-dihydroxydibenzo-p-dioxin-1,2-dione (II) and 2,3a,5,7-tetra-t-butyl-3a,10a-dihydro-8,10a-dihydroxybenzo[b]cyclopenta[f][1,4]dioxepin-1,10-dione (IV), respectively. The suggested position of substitution of the t-butyl group in 5-t-butylpyrogallol提出的证据表明4,6-二叔丁基-3-羟基-邻苯醌的黄色和白色二聚体是结构3,4a,6,8-四叔丁基-4a,10a-二氢-9,10a-二羟基二苯并-对二恶英-1,2-二酮(II)和2,3a,5,7-四叔丁基-3a,10a-二氢-8,10a-二羟基苯并[ b ]环戊[分别为f ] [1,4] dioxepin-1,10-dione(IV)。已经证实了叔丁基在5-叔丁基邻苯三酚中的取代位置,从而确定了5-叔丁基-3-羟基-邻苯醌的二聚体为5,10-二叔丁基- 2,7-二羟基三环-[5,3,1,1 2,6] dodeca-4,9-二烯-3,8,11,12-四酮(XIII)。在制备5-叔丁基间苯三酚中,使用了新的反应序列以原位连接叔丁基。
-
Synthesis and Functionalization of Inherently Chiral Tetraoxacalix[2]arene[2]pyridines作者:Shuai Pan、De-Xian Wang、Liang Zhao、Mei-Xiang WangDOI:10.1021/ol303019q日期:2012.12.21Inherently chiral tetraoxacalix[2]arene[2]pyridines containing C2 symmetry were synthesized efficiently from a macrocyclic condensation reaction of resorcinol derivatives with 2,6-dichloro-3-nitropyridine in a one-pot reaction manner, while tetraoxacalix[2]arene[2]pyridine with an ABCD-substitution pattern was prepared in a good yield by means of a stepwise fragment coupling approach. Postmacrocyclization
-
Myricetin, rosmarinic and carnosic acids as superior natural antioxidant alternatives to α-tocopherol for the preservation of omega-3 oils作者:Romain Guitard、Jean-François Paul、Véronique Nardello-Rataj、Jean-Marie AubryDOI:10.1016/j.foodchem.2016.06.038日期:2016.12transfer, number of radicals scavenged per antioxidant molecule, BDE and formation of antioxidant dimers from the primary radicals play an important role regarding the antioxidant activity of phenols. Based on this, it is finally shown that myricetin, rosmarinic and carnosic acids are more efficient than α-tocopherol and synthetic antioxidants for the preservation of omega-3 oils.
-
The oxidation of some pyrogallol and purpurogallin derivatives作者:A. Critchlow、E. Haslam、R.D. Haworth、P.B. Tinker、N.M. WaldronDOI:10.1016/0040-4020(67)85149-4日期:1967.1The oxidation products of a number of 4- and 5-monosubstituted and 4,6-disubstituted pyrogallols are discussed. 4-Alkylpyrogallols yield the corresponding 4′,7-dialkylpurpurogallins and the structures of the products resulting from the further oxidation of these compounds with alkaline hydrogen peroxide are elucidated. Oxidation of 5-mono and 4,6-dialkylpyrogallol derivatives yield characteristic white
-
PENAM DERIVATIVES FOR TREATING BACTERIAL INFECTIONS申请人:TenNor Therapeutics Limited公开号:US20210070774A1公开(公告)日:2021-03-11Novel iron chelating group conjugated penam derivatives described herein show antibacterial activity, and could be used as antibacterial agents or beta-lactamase inhibitors (BLIs) which are of value for application in combination with other antibacterial agents.
表征谱图
-
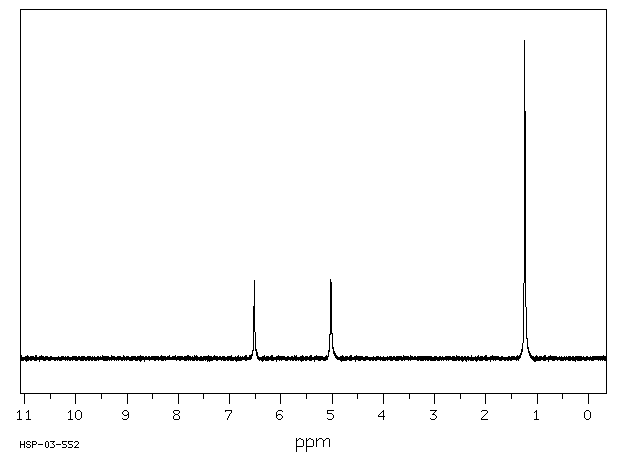
氢谱1HNMR
-
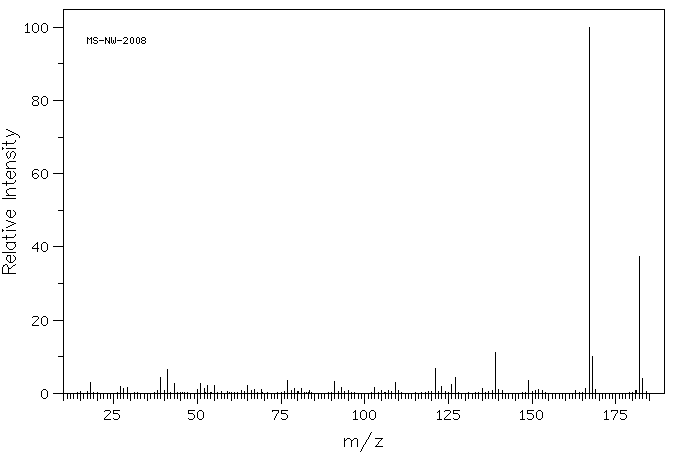
质谱MS
-
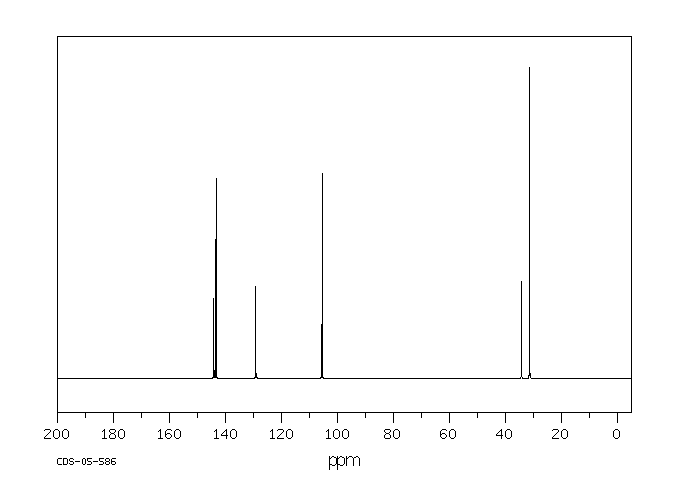
碳谱13CNMR
-
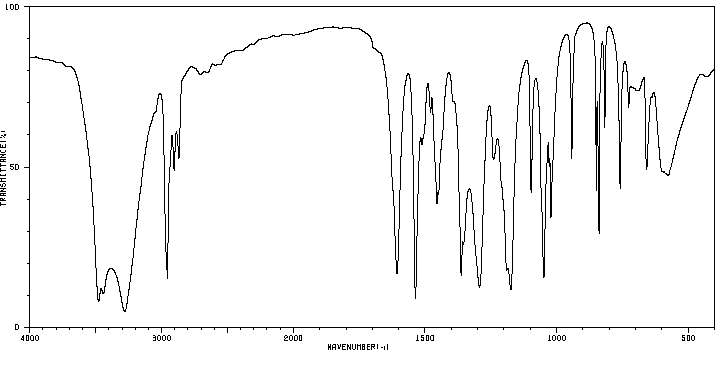
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚