胆固醇氢化肉桂酸盐 | 14914-99-9
分子结构分类
中文名称
胆固醇氢化肉桂酸盐
中文别名
氢化肉桂酸胆固醇酯
英文名称
cholesteryl β-phenylpropionate
英文别名
cholesteryl 3-phenylpropanoate;Cholesterol Hydrocinnamate;[(3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl] 3-phenylpropanoate
CAS
14914-99-9
化学式
C36H54O2
mdl
MFCD00003640
分子量
518.824
InChiKey
KPNKAGLPVPTLGB-ZOJFKXTHSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:111 °C
-
沸点:587.8±29.0 °C(Predicted)
-
密度:1.02±0.1 g/cm3(Predicted)
-
LogP:12.683 (est)
计算性质
-
辛醇/水分配系数(LogP):11
-
重原子数:38
-
可旋转键数:10
-
环数:5.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
储存条件:存放于惰性气体中;避免接触湿气(否则可能分解)。
SDS
氢化肉桂酸胆固醇酯
模块 1. 化学品
产品名称: Cholesterol Hydrocinnamate
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 氢化肉桂酸胆固醇酯
百分比: >95.0%(GC)
CAS编码: 14914-99-9
俗名: Cholesterol 3-Phenylpropionate , Hydrocinnamic Acid Cholesterol Ester , 3-
Phenylpropionic Acid Cholesterol Ester
分子式: C36H54O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
氢化肉桂酸胆固醇酯
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点:
111°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
氢化肉桂酸胆固醇酯
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
氢化肉桂酸胆固醇酯
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Cholesterol Hydrocinnamate
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 氢化肉桂酸胆固醇酯
百分比: >95.0%(GC)
CAS编码: 14914-99-9
俗名: Cholesterol 3-Phenylpropionate , Hydrocinnamic Acid Cholesterol Ester , 3-
Phenylpropionic Acid Cholesterol Ester
分子式: C36H54O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
氢化肉桂酸胆固醇酯
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点:
111°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
氢化肉桂酸胆固醇酯
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
氢化肉桂酸胆固醇酯
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Chemoselective epoxidation of cholesterol derivatives on a surface-designed molecularly imprinted Ru–porphyrin catalyst摘要:胆固醇衍生物的C5-C6环氧化具有高度的化学选择性,而不需要保护其他可氧化的官能团,在新设计的分子印迹Ru-卟啉催化剂上实现,该催化剂使用SiO2支撑。DOI:10.1039/c8cc00896e
-
作为产物:参考文献:名称:氟双相技术中用于酯交换反应的氟烷基二恶烷烷催化剂摘要:依靠氟双相技术,已经提出了新颖,实用的酯交换方法。氟烷基二氧杂环己烷烷催化剂能够通过使用比例为1的反应物酯和醇,在FC-72溶剂中进行酯交换反应,以提供100%的所需酯产率。该催化剂还可以在FC-72 /有机溶剂系统以及仅在甲苯中使用。可以使用许多带有各种官能团的酯和醇。催化剂可以完全回收再利用。更方便地,从反应混合物中分离出的FC-72中的催化剂溶液直接用于下一步反应。DOI:10.1002/1615-4169(200201)344:1<84::aid-adsc84>3.0.co;2-c
-
作为试剂:描述:dicyclohexyl fumarate 、 2,6-bis(decyloxy)anthracene 在 胆固醇氢化肉桂酸盐 、 cholesterol 2,4-dichlorobenzoate 作用下, 以 均三甲苯 为溶剂, 反应 48.0h, 生成 、参考文献:名称:双分子热反应的液晶控制。在液晶介质中富马酸酯与 2,6-二烷氧基蒽的高区域选择性环加成反应摘要:已经研究了液晶溶剂相控制 2,6-二烷氧基蒽与一系列富马酸酯在 130-180 °C 下进行双分子热反应的立体化学过程的能力,主要是关于溶质与溶剂介晶。对于 2,6-双(癸氧基)蒽与双(反式-4-环己基环己基)和胆甾醇反式-4-环己基环己基富马酸酯在 130-150 °C、胆甾醇 2 的模型热 [4 + 2] 环加成的情况, 4-二氯苯甲酸酯(CDCB)和双(4-戊氧基苯基)反式-1,4-环己烷二甲酸酯(BPCD)非常适合用作胆甾型和近晶型液晶溶剂,并导致优先形成具有极高区域选择水平的顺式异构体(同步/反≥ 20/1)。相比之下,具有密切相关结构的各向同性溶剂的异构体比例仅为 ≥ 3/1。溶质和溶剂介晶之间的结构相似性似乎在 c...DOI:10.1021/ja960282w
文献信息
-
Selective Construction of C−C and C=C Bonds by Manganese Catalyzed Coupling of Alcohols with Phosphorus Ylides作者:Xin Liu、Thomas WernerDOI:10.1002/adsc.202001209日期:2021.2.16manganese catalyzed coupling of alcohols with phosphorus ylides. The selectivity in the coupling of primary alcohols with phosphorus ylides to form carbon‐carbon single (C−C) and carbon‐carbon double (C=C) bonds can be controlled by the ligands. In the conversion of more challenging secondary alcohols with phosphorus ylides the selectivity towards the formation of C−C vs. C=C bonds can be controlled by the
-
Phosphorus oxychloride as an efficient coupling reagent for the synthesis of esters, amides and peptides under mild conditions作者:Hu Chen、Xunfu Xu、Liu Leo Liu、Guo Tang、Yufen ZhaoDOI:10.1039/c3ra42887g日期:——A mild method is described for the conversion of carboxylic acids into esters, amides, as well as peptides without racemization through carboxyl activation by the reagent combination of POCl3 and DMAP. Long chain alcohols could be converted to the corresponding ester in good yields. 31P NMR spectrum was used to detect phosphorus-containing intermediates in ongoing reactions directly, and a possible描述了一种温和的方法,可通过POCl 3和地图。长链醇可以良好的产率转化为相应的酯。31 P NMR光谱用于直接检测正在进行的反应中的含磷中间体,并基于这些结果提出了可能的机理。
-
Mechanistic studies of DCC/HOBt-mediated reaction of 3-phenylpropionic acid with benzyl alcohol and studies on the reactivities of ‘active ester’ and the related derivatives with nucleophiles作者:Md. Chanmiya Sheikh、Shunsuke Takagi、Toshiaki Yoshimura、Hiroyuki MoritaDOI:10.1016/j.tet.2010.07.011日期:2010.9extensive study for peptide synthesis, DCC-mediated esterification is left still unclear. Therefore, DCC- and DCC/HOBt-mediated reactions of 3-phenylpropionic acid (1) with benzyl alcohol were carried out under several mechanistic considerations. Further, in order to determine the reactivities of the so-called ‘active esters’ compounds changing the substituents bearing carbonyl and related derivatives group
-
Nickel-Catalyzed Esterification of Aliphatic Amides作者:Liana Hie、Emma L. Baker、Sarah M. Anthony、Jean-Nicolas Desrosiers、Chris Senanayake、Neil K. GargDOI:10.1002/anie.201607856日期:2016.11.21demonstrated that amides can be used in nickel‐catalyzed reactions that lead to cleavage of the amide C−N bond, with formation of a C−C or C−heteroatom bond. However, the general scope of these methodologies has been restricted to amides where the carbonyl is directly attached to an arene or heteroarene. We now report the nickel‐catalyzed esterification of amides derived from aliphatic carboxylic acids
-
Rh-catalyzed alkoxycarbonylation of unactivated alkyl chlorides作者:Peng Wang、Yaxin Wang、Helfried Neumann、Matthias BellerDOI:10.1039/d2sc04103k日期:——Crucial for this transformation is the addition of sodium iodide, which provides in situ more active alkyl iodides. In the presence of a Rh(I)-DPPP catalyst system diverse esters (81 examples) including industrially relevant acetates from chloro- and dichloromethane can be prepared in a straightforward manner in up to 95% isolated yield. The used ligand not only affects the selectivity of the carbonylation
表征谱图
-
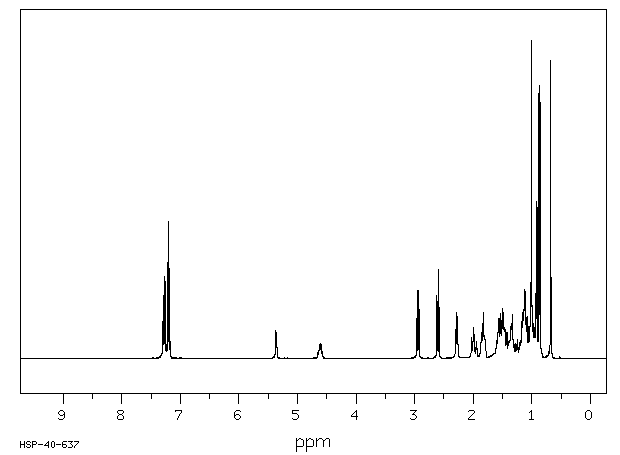
氢谱1HNMR
-
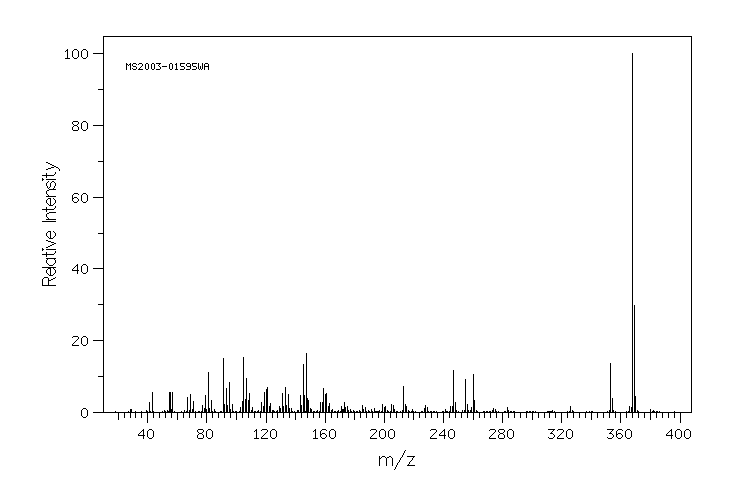
质谱MS
-
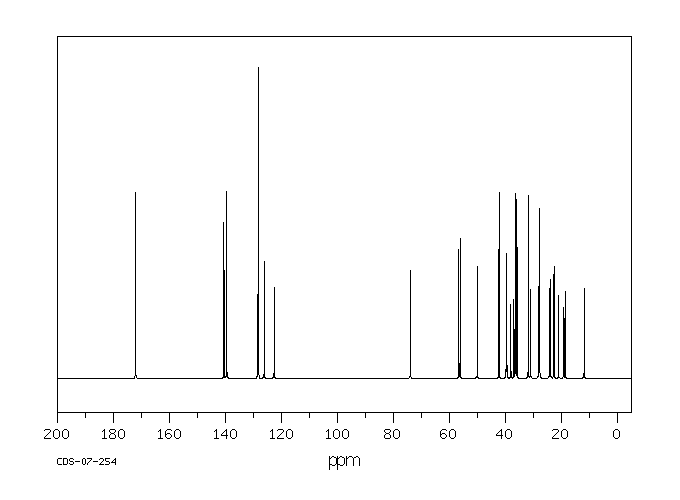
碳谱13CNMR
-
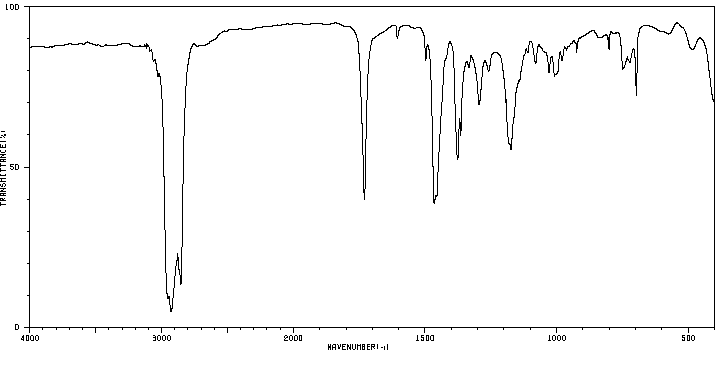
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β)-17,20:20,21-双[亚甲基双(氧基)]孕烷-3-酮
(5α)-2′H-雄甾-2-烯并[3,2-c]吡唑-17-酮
(3β,20S)-4,4,20-三甲基-21-[[[三(异丙基)甲硅烷基]氧基]-孕烷-5-烯-3-醇-d6
(25S)-δ7-大发酸
(20R)-孕烯-4-烯-3,17,20-三醇
(11β,17β)-11-[4-({5-[(4,4,5,5,5-五氟戊基)磺酰基]戊基}氧基)苯基]雌二醇-1,3,5(10)-三烯-3,17-二醇
齐墩果酸衍生物1
黄麻属甙
黄芪皂苷III
黄芪皂苷 II
黄芪甲苷 IV
黄芪甲苷
黄肉楠碱
黄果茄甾醇
黄杨醇碱E
黄姜A
黄夹苷B
黄夹苷
黄夹次甙乙
黄夹次甙乙
黄夹次甙丙
黄体酮环20-(乙烯缩醛)
黄体酮杂质EPL
黄体酮杂质1
黄体酮杂质
黄体酮杂质
黄体酮EP杂质M
黄体酮EP杂质G(RRT≈2.53)
黄体酮EP杂质F
黄体酮6-半琥珀酸酯
黄体酮 17alpha-氢过氧化物
黄体酮 11-半琥珀酸酯
黄体酮
麦角甾醇葡萄糖苷
麦角甾醇氢琥珀酸盐
麦角甾烷-6-酮,2,3-环氧-22,23-二羟基-,(2b,3b,5a,22R,23R,24S)-(9CI)
麦角甾烷-3,6,8,15,16-五唑,28-[[2-O-(2,4-二-O-甲基-b-D-吡喃木糖基)-a-L-呋喃阿拉伯糖基]氧代]-,(3b,5a,6a,15b,16b,24x)-(9CI)
麦角甾烷-26-酸,5,6:24,25-二环氧-14,17,22-三羟基-1-羰基-,d-内酯,(5b,6b,14b,17a,22R,24S,25S)-(9CI)
麦角甾-8-烯-3-醇
麦角甾-8,24(28)-二烯-26-酸,7-羟基-4-甲基-3,11-二羰基-,(4a,5a,7b,25S)-
麦角甾-7,22-二烯-3-酮
麦角甾-7,22-二烯-17-醇-3-酮
麦角甾-5,24-二烯-26-酸,3-(b-D-吡喃葡萄糖氧基)-1,22,27-三羟基-,d-内酯,(1a,3b,22R)-
麦角甾-5,22,25-三烯-3-醇
麦角甾-4,6,8(14),22-四烯-3-酮
麦角甾-1,4-二烯-3-酮,7,24-二(乙酰氧基)-17,22-环氧-16,25-二羟基-,(7a,16b,22R)-(9CI)
麦角固醇
麦冬皂苷D
麦冬皂苷D
麦冬皂苷 B