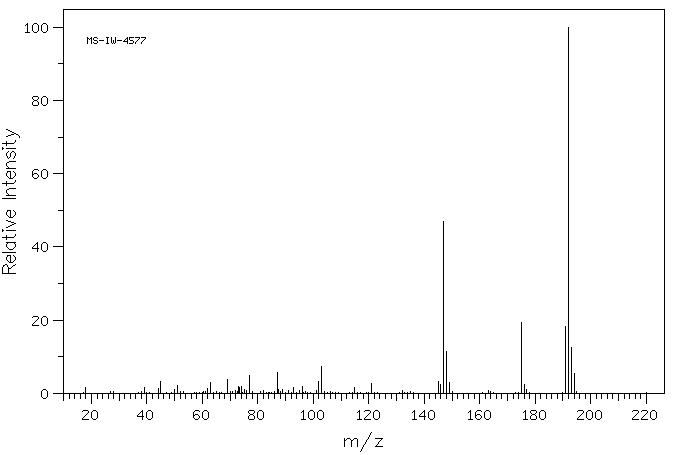
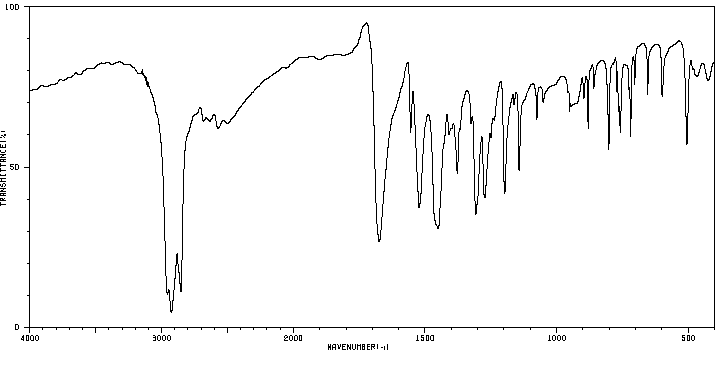
5-甲基-1-苯并噻吩-2-羧酸 | 1505-62-0
中文名称
5-甲基-1-苯并噻吩-2-羧酸
中文别名
——
英文名称
5-methyl-benzo[b]thiophene-2-carboxylic acid
英文别名
5-methyl-1-benzothiophene-2-carboxylic acid;5-methylbenzo[b]thiophene-2-carboxylic acid;2-carboxy-5-methylbenzo[b]thiophene;5-methyl-benzo[b]thiophene-2-carboxylic acid;5-Methyl-benzo[b]thiophen-2-carbonsaeure
CAS
1505-62-0
化学式
C10H8O2S
mdl
MFCD04969088
分子量
192.238
InChiKey
OUFWTHMQVXSBCF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:218.0-219.5 °C
-
沸点:386.1±22.0 °C(Predicted)
-
密度:1.355±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:65.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
安全说明:S26
-
危险类别码:R36/37/38
-
海关编码:2934999090
SDS
| Name: | 5-Methyl-1-benzothiophene-2-carboxylic acid Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 1505-62-0 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1505-62-0 | 5-Methyl-1-benzothiophene-2-carboxylic | 97+% | unlisted |
Risk Phrases: 20/21/22 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Harmful if inhaled. Causes respiratory tract irritation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1505-62-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 222 - 224 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H8O2S
Molecular Weight: 192.24
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Dust generation.
Incompatibilities with Other Materials:
Amines, strong oxidizing agents, strong bases.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1505-62-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
5-Methyl-1-benzothiophene-2-carboxylic acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 22 Do not breathe dust.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
WGK (Water Danger/Protection)
CAS# 1505-62-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1505-62-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1505-62-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-甲基-苯并[B]噻吩-2-羧酸甲酯 methyl 5-methylbenzo[b]thiophene-2-carboxylate 82787-69-7 C11H10O2S 206.265 —— ethyl 5-methylbenzo[b]thiophene-2-carboxylate 101219-39-0 C12H12O2S 220.292 —— 2-acetyl-5-methylbenzo[b]thiophene 2046-77-7 C11H10OS 190.266 5-甲基苯并噻吩 5-methylthianaphthene 14315-14-1 C9H8S 148.229 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-甲基-苯并[B]噻吩-2-羧酸甲酯 methyl 5-methylbenzo[b]thiophene-2-carboxylate 82787-69-7 C11H10O2S 206.265 —— methyl 5-(bromomethyl)benzo[b]thiophene-2-carboxylate 82787-70-0 C11H9BrO2S 285.161 —— tert-butyl 5-methyl-1-benzothiophene-2-carboxylate 1427753-41-0 C14H16O2S 248.346 —— 5-Imidazol-1-ylmethyl-benzo[b]thiophene-2-carboxylic acid 82788-51-0 C13H10N2O2S 258.301 —— 5-(1-imidazolylmethyl)benzo[b]thiophene-2-carboxylic acid methyl ester 82787-71-1 C14H12N2O2S 272.327 —— 5-methyl-3-fluorobenzo[b]thiophene-2-carboxylic acid 348080-96-6 C10H7FO2S 210.229 5-甲基苯并噻吩 5-methylthianaphthene 14315-14-1 C9H8S 148.229
反应信息
-
作为反应物:描述:5-甲基-1-苯并噻吩-2-羧酸 在 盐酸 、 N-溴代丁二酰亚胺(NBS) 、 偶氮二异丁腈 、 sodium hydride 作用下, 以 四氯化碳 为溶剂, 反应 9.0h, 生成 5-(1-imidazolylmethyl)benzo[b]thiophene-2-carboxylic acid methyl ester参考文献:名称:Selective thromboxane synthetase inhibitors. 3. 1H-Imidazol-1-yl-substituted benzo[b]furan-, benzo[b]thiophene- and indole-2- and 3-carboxylic acids摘要:The preparation of a series of 1H-imidazol-1-yl-substituted benzo[b]furan-, benzo[b]thiophene-, and indolecarboxylic acids is described. Most of the compounds were potent inhibitors of TxA2 synthetase in vitro, and the distance between the imidazole and carboxylic acid groups was found to be important for optimal potency. The most potent compound in vivo was 6-(1H-imidazol-1-ylmethyl)-3-methylbenzo[b]thiophene-2-carboxylic acid (71), which, in conscious dogs, showed a similar profile of activity to that of dazoxiben (1).DOI:10.1021/jm00159a012
-
作为产物:描述:参考文献:名称:Krollpfeiffer et al., Justus Liebigs Annalen der Chemie, 1950, vol. 566, p. 139,145摘要:DOI:
文献信息
-
Substituted imidazoles, pharmaceutical compositions containing these,申请人:Hoechst Aktiengesellschaft公开号:US05350751A1公开(公告)日:1994-09-27The invention relates to compounds of the formula I ##STR1## in which X, Y and Z are identical or different and are N or CR.sup.2, R.sup.1 and R.sup.2 are as defined in the description, L is an alkylene radical, q is 0 or 1, and A is the radical of a fused heterobicyclic compound. The invention furthermore relates to a process for preparing the said compounds, agents containing these, and the use thereof in the treatment of high blood pressure.该发明涉及公式I的化合物##STR1##其中X、Y和Z相同或不同,为N或CR.sup.2,R.sup.1和R.sup.2如描述中定义,L为烷基基团,q为0或1,A为融合杂环化合物的基团。该发明还涉及制备所述化合物的方法,含有这些化合物的药剂,以及在治疗高血压中的使用。
-
Construction of Thienothiophene and Thienofuran Ring Systems via Ring Expansion of Difluorothiiranes Generated from Dithioesters作者:Kohei Fuchibe、Ibuki Mukohara、Atsushi Yamada、Daisuke Miyazaki、Ryo Takayama、Junji IchikawaDOI:10.1021/acs.orglett.1c03805日期:2022.1.14The reaction of aryl thiophene-2-carbodithioates or thiophene-3-carbodithioates with difluorocarbene generated from BrCF2CO2Li/molecular sieves 4A produced arylsulfanylated 2,2-difluoro-3-thienylthiiranes. In the presence of lithium ion, the thiirane intermediates underwent ring expansion followed by HF elimination, leading to fluorinated thieno[3,2-b]thiophenes or thieno[2,3-b]thiophenes. The reactions
-
[EN] SPIROCYCLE COMPOUNDS AND METHODS OF MAKING AND USING SAME<br/>[FR] COMPOSÉS SPIROCYCLIQUES ET PROCÉDÉS DE PRÉPARATION ET D'UTILISATION DE CEUX-CI申请人:ABIDE THERAPEUTICS INC公开号:WO2019046318A1公开(公告)日:2019-03-07Provided herein are compounds and compositions useful as modulators of MAGL. Furthermore, the subject compounds and compositions are useful for the treatment of pain.本文提供的化合物和组合物可用作MAGL的调节剂。此外,这些化合物和组合物可用于治疗疼痛。
-
Benzimidazole derivatives申请人:——公开号:US20040010004A1公开(公告)日:2004-01-15A benzimidazole derivative or its medically acceptable salt, represented by the following formula (1), that is a human chymase activity inhibitor capable of being applied clinically: 1 wherein, R 1 and R 2 represent a hydrogen atom, an alkyl group or an alkoxy group, etc., A represents an alkylene group or an alkenylene group, E represents —COOR 3 , —SO 3 R 3 , —CONHR 3 or —SO 2 NHR 3 , etc., G represents an alkylene group, M represents a single bond or —S(O) m . J represents a heterocyclic group, and X represents —CH═ or a nitrogen atom.
-
Benzimidazole derivative申请人:Tsuchiya Naoki公开号:US20050267148A1公开(公告)日:2005-12-01The present invention is a thiobenzimidazole derivative represented by the following formula (1) or a medically acceptable salt thereof wherein said thiobenzimidazole derivative and a medically acceptable salt thereof have a potent activity of inhibiting human chymase. Thus, they are potential preventive and/or therapeutic agents clinically applicable to various diseases in which human chymase is involved.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐留通钠
齐留通相关物质A
齐留通亚砜
齐留通-d4
齐留通
雷洛昔芬杂质
邻联甲苯胺砜
试剂4,8-Bis(3,5-dioctyl-2-thienyl)-2,6-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[1,2-b:4,5-b']dithiophene
试剂1,1'-[4,8-Bis[4-(2-ethylhexyl)-3,5-difluorophenyl]benzo[1,2-b:4,5-b']dithiophene-2,6-diyl]bis[1,1,1-trimethylstannane]
苯并噻吩-7-醇
苯并噻吩-4-硼酸频哪醇酯
苯并噻吩-3-羧酸甲酯
苯并噻吩-3-硼酸
苯并噻吩-2-羰酰氯
苯并噻吩-2-羧酸肼
苯并噻吩-2-羧酸
苯并噻吩-2-硼酸
苯并噻吩-2-氨基甲酸叔丁酯
苯并噻吩
苯并[c]噻吩
苯并[b]噻吩-7-胺
苯并[b]噻吩-7-羧酸乙酯
苯并[b]噻吩-7-甲醛
苯并[b]噻吩-7-甲腈
苯并[b]噻吩-6-醇
苯并[b]噻吩-6-胺
苯并[b]噻吩-6-羧酸乙酯
苯并[b]噻吩-6-羧酸
苯并[b]噻吩-6-甲腈
苯并[b]噻吩-5-甲腈,2-甲酰基-
苯并[b]噻吩-5-甲磺酰氯
苯并[b]噻吩-4-羧酸甲酯
苯并[b]噻吩-4-羧酸
苯并[b]噻吩-4-甲醛
苯并[b]噻吩-4-甲腈
苯并[b]噻吩-4-基甲醇
苯并[b]噻吩-3-胺盐酸盐
苯并[b]噻吩-3-胺
苯并[b]噻吩-3-羧酸-(2-二烯丙基氨基乙酯)
苯并[b]噻吩-3-硼酸频哪酯
苯并[b]噻吩-3-甲醛肟
苯并[b]噻吩-3-甲酰胺
苯并[b]噻吩-3-基乙酸酯
苯并[b]噻吩-3-乙酸
苯并[b]噻吩-3-乙酰氯
苯并[b]噻吩-3-乙腈
苯并[b]噻吩-2-胺盐酸盐
苯并[b]噻吩-2-羧酸6-氨基-3-氯-甲酯
苯并[b]噻吩-2-羧酸,5-氯-3-(1-甲基乙氧基)-
苯并[b]噻吩-2-羧酸,3-羟基-5-甲氧基-,甲基酯