5-甲基-3-苯基-1,3-恶唑烷 | 73861-82-2
中文名称
5-甲基-3-苯基-1,3-恶唑烷
中文别名
——
英文名称
5-methyl-3-phenyl-oxazolidine
英文别名
5-Methyl-3-phenyl-oxazolidin;5-Methyl-3-phenyl-1,3-oxazolidine
CAS
73861-82-2
化学式
C10H13NO
mdl
——
分子量
163.219
InChiKey
VJPMKAHLPYDCHH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:12.5
-
氢给体数:0
-
氢受体数:2
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 1-(N-(methyl)phenylamino)propan-2-ol 3233-03-2 C10H15NO 165.235
反应信息
-
作为反应物:描述:参考文献:名称:Lapshova, A. A.; Zorin, V. V.; Zlotskii, S. S., Journal of Organic Chemistry USSR (English Translation), 1980, vol. 16, p. 325 - 330摘要:DOI:
-
作为产物:描述:参考文献:名称:基于醛与氨基醇缩合合成含(η6-芳烃)三羰基铬基团的新型1,3-恶唑烷和1,3-恶嗪烷摘要:研究了含苯基或(η6-芳烃)三羰基铬取代基的β-和γ-氨基醇与甲醛、乙醛、苯甲醛和(η6-苯甲醛)三羰基铬的缩合反应。得到的 1,3-恶唑烷和 1,3-恶嗪烷产物以纯形式分离并通过不同的物理化学方法进行鉴定。证明了(η6-芳烃)三羰基铬部分对反应过程的影响。DOI:10.1007/s11172-018-2153-0
文献信息
-
One-Pot Synthesis of Ethyl 3-(2-Hydroxyalkyl)aminoalkanoates by Ring Opening of 1,3-Oxazolidines Using Reformatsky Reagent作者:Tomihiro Nishiyama、Hiroshi Kishi、Kouji Kitano、Fukiko YamadaDOI:10.1246/bcsj.67.1765日期:1994.6Oxazolidines, obtained by the condensation of aldehydes with 2-anilino-1-alkanols, react with the Reformatsky reagent derived from ethyl bromoacetate under mild reaction conditions to afford ethyl 3-(2-hydroxyalkyl)aminoalkanoates.
-
Reaction of phenyl-containing N-substituted 1,3-oxazolidines and 1,3-oxazinanes with triammine(tricarbonyl)chromium作者:A. N. Artemov、E. V. Sazonova、N. A. Aksenova、G. K. Fukin、A. V. Cherkasov、V. I. Faerman、N. Yu. GrishinaDOI:10.1007/s11172-019-2590-4日期:2019.8New heterocyclic compounds with phenyl chromium tricarbonyl substituents were synthesized by the reaction of triammine(tricarbonyl)chromium with phenyl-substituted 1,3-oxazacy-cloalkanes bearing acetyl, tert-butyloxycarbonyl, or phenyl group at the nitrogen atom. The resulting compounds were isolated in the individual state and characterized by physicochemical methods of analysis.
-
201. Some compounds related to the aromatic “nitrogen mustards.”作者:George A. R. Kon、John J. RobertsDOI:10.1039/jr9500000978日期:——
-
Petrow et al., Zhurnal Obshchei Khimii, 1953, vol. 23, p. 1771,1773; engl. Ausg. S. 1869, 1871作者:Petrow et al.DOI:——日期:——
-
Unexpected 1,3-oxazolidine formation in the attempted oxidation of N-aryl-N-methyl substituted β-amino alcohols using pyridinium dichromate作者:Jari T. Yli-Kauhaluoma、Curtis W. Harwig、Paul Wentworth、Kim D. JandaDOI:10.1016/s0040-4039(98)00289-5日期:1998.41,3-Oxazolidines were obtained from the reaction of N-methyl substituted beta-amino alcohols with pyridinium dichromate in dichloromethane. A single electron transfer mechanism. SET, is proposed to account for the formation of the 1,3-oxazolidines. (C) 1998 Elsevier Science Ltd. All rights reserved.
表征谱图
-
氢谱1HNMR
-
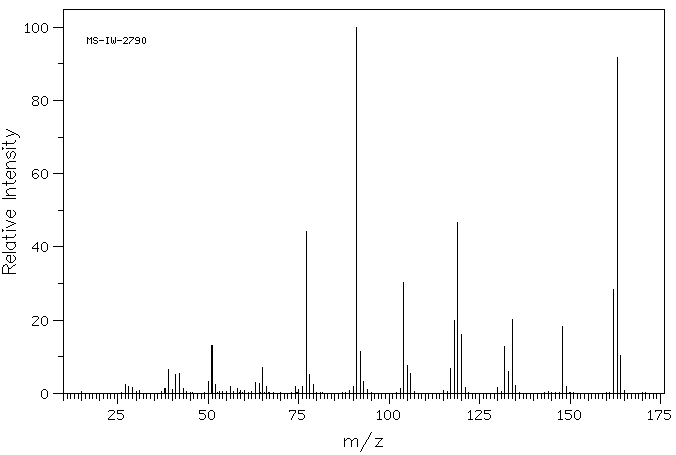
质谱MS
-
碳谱13CNMR
-
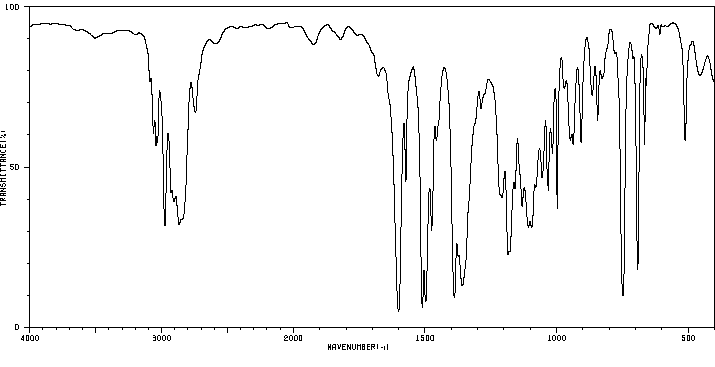
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷