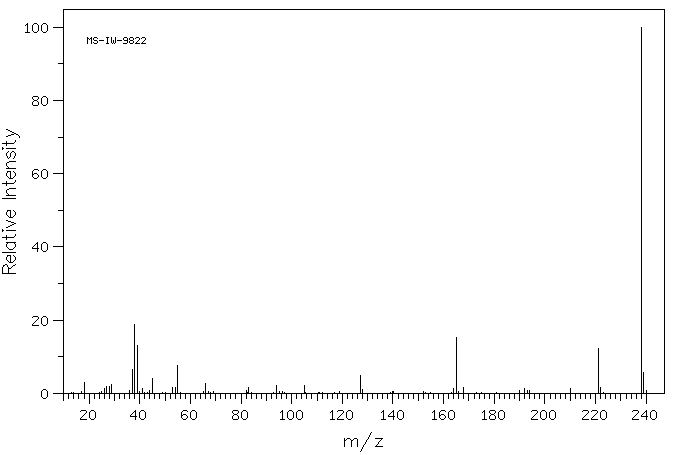
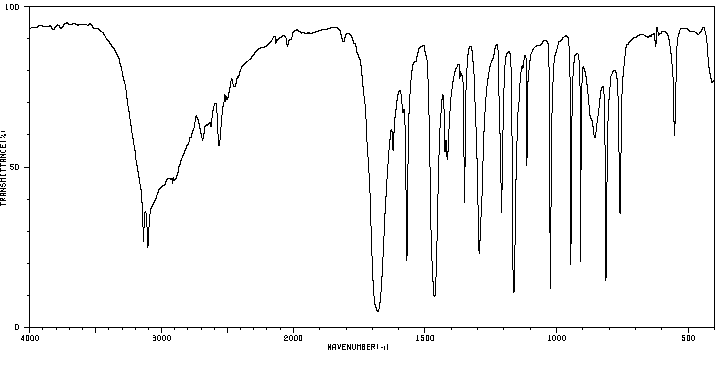
5-碘-2-糠酸 | 18614-11-4
中文名称
5-碘-2-糠酸
中文别名
——
英文名称
5-iodofuran-2-carboxylic acid
英文别名
5-iodo-2-furoic acid;5-iodo-furan-2-carboxylic acid;5-Jod-furan-2-carbonsaeure;5-Iod-2-furancarbonsaeure;5-Iodfuran-2-carbonsaeure;2-Iod-5-carboxy-furan
CAS
18614-11-4
化学式
C5H3IO3
mdl
MFCD02180668
分子量
237.982
InChiKey
UKRYIKCRRFOEOD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:192 °C
-
沸点:326.4±27.0 °C(Predicted)
-
密度:2.227±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:50.4
-
氢给体数:1
-
氢受体数:3
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
危险性防范说明:P305+P351+P338
-
危险性描述:H319
SDS
| Name: | 5-Iodo-2-furoic acid 97% Material Safety Data Sheet |
| Synonym: | 5-Iodofuran-2-carboxylic aci |
| CAS: | 18614-11-4 |
Synonym:5-Iodofuran-2-carboxylic aci
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 18614-11-4 | 5-Iodo-2-furoic acid | 97% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 18614-11-4: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 195 - 196 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C5H3IO3
Molecular Weight: 238
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, hydrogen iodide, iodine, acrid smoke and fumes.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 18614-11-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
5-Iodo-2-furoic acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 18614-11-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 18614-11-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 18614-11-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 糠酸(呋喃甲酸) 2-Furoic acid 88-14-2 C5H4O3 112.085 5-碘-2-呋喃甲醛 5-iodofuran-2-carbaldehyde 2689-65-8 C5H3IO2 221.982 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-碘呋喃-2-羧酸甲酯 methyl 5-bromo-2-furoate 2527-98-2 C6H5IO3 252.008 2,5-呋喃二甲酸 furan-2,5-dicarboxylic acid 3238-40-2 C6H4O5 156.095
反应信息
-
作为反应物:描述:参考文献:名称:Aroylthioureas: new organic ionophores for heavy-metal ion selective electrodes摘要:合成了46种不同取代基的噻唑衍生物(芳酰噻唑),并研究了它们在离子选择电极(ISEs)中的离子载体潜力。根据相应的重金属ISE参数,考虑了结构因素。作为离子载体,某些1-呋喃酰基-3-取代噻唑(系列2)在Pb(II)、Hg(II)和Cd(II) ISE中表现出最佳性能。系列2中的强内氢键使得配体只能通过CS基团进行相互作用。呋喃和苯环上的取代基导致在膜增塑剂中溶解度低。3-烷基取代的呋喃酰噻唑提高了溶解度,但随着链长的增加增强了氧化过程。在分析取代基对ISE选择性的影响时,考虑了新的X射线衍射(XRD)结构和理论DFT计算。这些新离子载体因其稳定性、简单合成和由于取代改变硫结合能力的易修改性而具有优势。DOI:10.1039/b102029n
-
作为产物:描述:bis-(5-formyl-[2]furyl)-mercury 在 1,4-二氧六环 、 sodium hydroxide 、 乙醇 、 双氧水 、 碘 、 mercury dichloride 作用下, 生成 5-碘-2-糠酸参考文献:名称:SOME REACTIONS OF 5-CHLOROMERCURI-2-FURFURYL ALCOHOL1摘要:DOI:10.1021/jo01201a015
文献信息
-
Radioiodinated ?1-adrenergic receptor ligands作者:Terence G. Hamill、H. Donald Burns、Raymond E. GibsonDOI:10.1002/jlcr.433日期:2001.1The synthesis and preliminary characterization of three radioiodinated α1-adrenergic receptor ligands [125I]5a–c are described. These tracers are analogs of L-760,478, 1b, itself an analog of prazosin, 1a. The highest affinity tracer, [125I]5a, has six fold higher affinity for the α1 receptor subtypes than prazosin. These ligands could be useful for autoradiographic studies of the α1 receptor subtypes. Copyright © 2001 John Wiley & Sons, Ltd.
-
一种2,5-呋喃二甲酸的合成工艺
-
Kotschetkow; Nifant'ew, Vestnik Moskovskogo Universiteta, 1958, vol. 13, # 5, p. 119,121作者:Kotschetkow、Nifant'ewDOI:——日期:——
-
Intramolecular Cyclization Reactions of 5-Halo- and 5-Nitro-Substituted Furans作者:Kenneth R. Crawford、Scott K. Bur、Christopher S. Straub、Albert PadwaDOI:10.1021/ol035233k日期:2003.9.1[GRAPHICS]Intramolecular cyclization reactions of 5-halo- and 5-nitro-substituted furanylamides were examined. The 2-alkoxy-5-bromofuran derivative 2 produced the rearranged dihydroquinone 6 (36%), a product from the rearrangement of the intermediate oxabicycle 3. The 5-halo substituted furoyl amide 18 was converted to the polyfunctional oxabicycle 20 in 82% yield and at a much faster rate than the unsubstituted furanyl system 17. The 5-nitro-substituted furfuryl amide 33b underwent an unusual isomerization-cyclization reaction under microwave conditions to provide 1,4-dihydro-2H-benzo[4,5]furo[2,3-c]pyridin-3-one 34.
-
Nasarowa, Zhurnal Obshchei Khimii, 1955, vol. 25, p. 539,541; engl. Ausg. S. 509, 511作者:NasarowaDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
除草醚
锡烷,三丁基[(2-呋喃基羰基)氧代]-
醋糠硫胺
醋呋三嗪
酪氨酰-甘氨酰-色氨酰-蛋氨酰-门冬氨酰-苯基丙氨酰-甘氨酸
苯胺,N-[6-乙氧基-2,3-二(4-甲氧苯基)-4H-吡喃-4-亚基]-4-甲基-
糠酸(呋喃甲酸)
糠酸異戊酯
糠酸烯丙酯
碘化溴刚
硫代糠酸甲酯
硝基呋喃杂质
硝呋隆
硝呋醛肟标准品
硝呋达齐
硝呋美隆
硝呋维啶
硝呋立宗
硝呋甲醚
硝呋烯腙盐酸盐
硝呋烯腙
硝呋替莫
硝呋拉定
硝呋拉嗪
硝呋太尔杂质B
硝呋太尔杂质33
硝呋噻唑
硝呋吡醇
硝呋乙宗
盐酸呋喃它酮
盐酸呋喃他酮
疏呋那登
甲基7-[5-乙酰氨基-4-[(2-溴-4,6-二硝基苯基)偶氮]-2-甲氧苯基]-3-羰基-2,4,10-三氧杂-7-氮杂十一烷-11-酸酯
甲基5-溴-3-甲基-2-糠酸酯
甲基5-乙酰氨基-2-糠酸酯
甲基5-{[(氯乙酰基)氨基]甲基}-2-糠酸酯
甲基5-(甲氧基甲基)-2-甲基呋喃-3-羧酸酯
甲基5-(溴甲基)-4-(氯甲基)-2-糠酸酯
甲基5-(乙氧基甲基)-2-甲基-3-糠酸酯
甲基5-({[5-(三氟甲基)-2-吡啶基]硫代}甲基)-2-糠酸
甲基5-(4-甲酰基苯基)-2-糠酸酯
甲基5-(3-甲酰基苯基)-2-糠酸酯
甲基4-甲基-3-糠酸酯
甲基4-溴-5-甲基-2-糠酸酯
甲基4-乙酰基-5-甲基-2-糠酸酯
甲基4,6-二氯-3-(二乙基氨基)呋喃并[3,4-c]吡啶-1-羧酸酯
甲基3-羟基呋喃并[3,2-b]吡啶-2-羧酸酯
甲基3-甲酰基-2-糠酸酯
甲基3-氨基呋喃并[2,3-b]吡啶-2-羧酸酯
甲基3-氨基-5-(2-甲基-2-丙基)-2-糠酸酯