色酚AS-BO | 132-68-3
中文名称
色酚AS-BO
中文别名
3-羟基-N-(1-萘基)-2-萘甲酰胺;2-羟基-3-萘甲酸-1-萘酰胺;冰染偶氮组分4;纳夫妥AS-BO;N-萘-1-基-3-羟基萘-2-甲酰胺;2-羟基-3-萘甲酰基-1-萘胺
英文名称
naphthol ASBO
英文别名
3-hydroxy-N-(naphthyl)-2-naphthylcarboxamide;3-hydroxy-N-naphthyl-2-naphthamide;naphthol ASSW;3-hydroxy-naphthalene-2-carboxylic acid naphthalen-1-ylamide;3-hydroxy-[2]naphthoic acid-[1]naphthylamide;3-Hydroxy-[2]naphthoesaeure-[1]naphthylamid;3-Hydroxy-N-1-naphthyl-2-naphthamide;3-hydroxy-N-naphthalen-1-ylnaphthalene-2-carboxamide
CAS
132-68-3
化学式
C21H15NO2
mdl
——
分子量
313.356
InChiKey
QGZGJNPVHADCFM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:222~223℃
-
沸点:479.3±28.0 °C(Predicted)
-
密度:1.333±0.06 g/cm3(Predicted)
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):5.5
-
重原子数:24
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:49.3
-
氢给体数:2
-
氢受体数:2
安全信息
-
RTECS号:QJ1897300
-
海关编码:2924299090
-
储存条件:存储条件:室温下密封保存。
SDS
3-Hydroxy-N-(1-naphthyl)-2-naphthamide Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 3-Hydroxy-N-(1-naphthyl)-2-naphthamide
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 3-Hydroxy-N-(1-naphthyl)-2-naphthamide
Percent: ....
CAS Number: 132-68-3
Synonyms: 2-Hydroxy-3-naphthoic Acid 1-Naphthylamide , Naphthol AS-BO , Azoic Coupling
Component 4
C21H15NO2
Chemical Formula:
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
3-Hydroxy-N-(1-naphthyl)-2-naphthamide
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Slightly pale yellow red
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
3-Hydroxy-N-(1-naphthyl)-2-naphthamide
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
ipr-rat LD50:7320 mg/kg
Acute Toxicity:
orl-rat LD:>12 g/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
QJ1897300
RTECS Number:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
3-Hydroxy-N-(1-naphthyl)-2-naphthamide
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 3-Hydroxy-N-(1-naphthyl)-2-naphthamide
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 3-Hydroxy-N-(1-naphthyl)-2-naphthamide
Percent: ....
CAS Number: 132-68-3
Synonyms: 2-Hydroxy-3-naphthoic Acid 1-Naphthylamide , Naphthol AS-BO , Azoic Coupling
Component 4
C21H15NO2
Chemical Formula:
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
3-Hydroxy-N-(1-naphthyl)-2-naphthamide
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Slightly pale yellow red
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
3-Hydroxy-N-(1-naphthyl)-2-naphthamide
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
ipr-rat LD50:7320 mg/kg
Acute Toxicity:
orl-rat LD:>12 g/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
QJ1897300
RTECS Number:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
3-Hydroxy-N-(1-naphthyl)-2-naphthamide
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质
浅米色粉末,溶于二甲苯,在烧碱溶液中呈黄色。不溶于水和碳酸钠溶液。在氢氧化钠溶液中为黄色,在空气中稳定。
色酚 AS-BO(N-萘-1-基-3-羟基萘-2-甲酰胺)主要用于棉纤维染色和棉布印花打底剂,也适用于维纶、黏胶纤维、蚕丝及锦纶的染色。与红色基 B 配合染紫酱色,具有较好的日晒牢度。N-萘-1-基-3-羟基萘-2-甲酰胺主要用作棉纤维染色和印花的打底剂,并作为有机颜料中间体及制造快色素的原料。该产品适用于棉纱、棉织物、针织物、灯芯绒、聚乙烯醇、黏胶、丝与锦纶等纤维染色,一般不用于印花。其对棉花有较高的亲合力和较强的偶联能力,可制成速成颜料、中性颗粒颜料及有机颜料。
生产方法以 2,3-酸和 1-萘胺为原料,首先将 2,3-酸制成钠盐,然后在三氯化磷存在下与 1-萘胺缩合。经中和、蒸馏、过滤、洗涤及干燥得成品。
具体步骤如下:
- 在成盐锅中加入氯苯4000-5000升、30%氢氧化钠500升、2,3-酸630公斤(以100%计)。升温脱水,至134-135℃,蒸出液透明无水为终点。料液体积约为2800-3000升。
- 将上述成盐溶液压入缩合锅中,冷却至90℃时加入1-萘胺479公斤(以100%计),继续冷却至67℃左右,在2小时内均匀加入三氯化磷-氯苯混合液(由230公斤三氯化磷与无水氯苯配成的55%-60%混合液)。加完后温度为118-120℃,保温2小时。
- 在蒸馏釜中加入水1000升、30%氢氧化钠330升,然后压入上述缩合液,搅拌15分钟,控制pH=8-8.5。随后通入直接蒸汽蒸氯苯,直至蒸出液澄清无氯苯为止。再加入90℃以上的热水洗涤,至滤液澄清,脱水、干燥得成品约960公斤。
N-萘-1-基-3-羟基萘-2-甲酰胺亦可通过2-羟基-3-萘甲酸与甲萘胺在三氯化磷存在下缩合而成。以2,3-酸为原料,用氯苯与烧碱成盐、脱水,再与甲萘胺和三氯化磷缩合,经中和、蒸馏、过滤、洗涤及干燥而得。
原料消耗(kg/t):
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-(1-Naphthalenyl)-3-[(6,7-dimethoxy-4-quinolyl)oxy]-2-naphthamide —— C32H24N2O4 500.554
反应信息
-
作为反应物:参考文献:名称:The Synthesis of Some 1-Nitroso and 1-Amino-2-Hydroxy-3-naphthoic Acid Aryl Amides摘要:DOI:10.1021/ja01164a517
-
作为产物:描述:参考文献:名称:DE506837摘要:公开号:
文献信息
-
Pigment compositions for solvent and water-based ink systems and the methods for producing them申请人:HOECHST CELANESE CORPORATION公开号:EP0592907A1公开(公告)日:1994-04-20This invention is an azo pigment composition containing a water insoluble metal salt of a water soluble polymer; a method of preparing said composition and ink compositions prepared from said azo pigment compositions.
-
Azo dyestuff intermediate nitro- or aminobenzenes ring-substituted by a申请人:Sterling Drug Inc.公开号:US04146558A1公开(公告)日:1979-03-27Water-soluble cationic dyestuffs of the formulae ##STR1## WHEREIN R.sup.0 is hydrogen, lower-alkyl or hydroxy-lower-alkyl; R.sup.1 is lower-alkyl, lower-alkenyl or hydroxy-lower-alkyl; R.sup.2 is lower-alkyl, lower-alkenyl, hydroxy-lower-alkyl or -(lower-alkylene)-NR.sup.0 Y or R.sup.1 and R.sup.2 together with the nitrogen atom, are pyrrolidino, piperidino or 4-lower-alkanoyl piperazino; Y is hydrogen or ##STR2## wherein R is hydrogen, lower-alkyl, lower-alkenyl, phenyl or phenyl-lower-alkyl; A is a member selected from the group consisting of an aromatic azo dyestuff residue attached to the quaternary ammonium nitrogen atom through a lower-alkylene bridge. K is a small integer whose value is dependent on the nature of A such that it has a range from one to two; R.sup.8 is lower-alkyl, lower-alkenyl or hydroxy-lower-alkyl; R.sup.9 is lower-alkyl, hydroxy-lower-alkyl or NH.sub.2 ; R.sup.10 is lower-alkyl or lower-alkenyl; A.sup.1 is an aromatic azo dyestuff residue attached to the quaternary ammonium nitrogen atom through a lower-alkylene bridge; G is a small integer whose value is dependent on the nature of A.sup.1 such that it has a range from one to two; R.sup.8 ' is lower-alkyl; R.sup.9 ' is lower-alkyl, lower-alkenyl or hydroxy-lower-alkyl; R.sup.10 ' is lower-alkyl, lower-alkenyl or hydroxy-lower-alkyl or R.sup.9 ' and R.sup.10 ' together with the nitrogen atom are morpholino; A.sup.2 is an aromatic azo dyestuff residue attached to the quaternary ammonium nitrogen atom through a lower-alkylene bridge; h is a small integer whose value is dependent on the nature of A.sup.2 such that it has a range from one to two; and An is an anion are particularly useful for coloring natural fibers, synthetic fiber-forming materials and cellulosic materials. These dyes dye cotton and paper various shades of stable yellows, reds and oranges. The dyeing from these dyes on paper are less prone to bleed and have a high degree of color discharge when bleached with hypochlorite or chlorine bleaches.水溶性阳离子染料的化学式为##STR1## 其中R.sup.0为氢、较低烷基或羟基较低烷基;R.sup.1为较低烷基、较低烯烃基或羟基较低烷基;R.sup.2为较低烷基、较低烯烃基、羟基较低烷基或-(较低烷基)-NR.sup.0 Y或R.sup.1和R.sup.2与氮原子一起,为吡咯烷基、哌啶基或4-较低酰基哌嗪基;Y为氢或##STR2## 其中R为氢、较低烷基、较低烯烃基、苯基或苯基较低烷基;A为从芳香偶氮染料残基中选择的一种,通过较低烷基桥连接到季铵氮原子。K是一个小整数,其值取决于A的性质,范围为一到二;R.sup.8为较低烷基、较低烯烃基或羟基较低烷基;R.sup.9为较低烷基、羟基较低烷基或NH.sub.2;R.sup.10为较低烷基或较低烯烃基;A.sup.1为从芳香偶氮染料残基中选择的一种,通过较低烷基桥连接到季铵氮原子;G是一个小整数,其值取决于A.sup.1的性质,范围为一到二;R.sup.8 '为较低烷基;R.sup.9 '为较低烷基、较低烯烃基或羟基较低烷基;R.sup.10 '为较低烷基、较低烯烃基或羟基较低烷基或R.sup.9 '和R.sup.10 '与氮原子一起为吗啉基;A.sup.2为从芳香偶氮染料残基中选择的一种,通过较低烷基桥连接到季铵氮原子;h是一个小整数,其值取决于A.sup.2的性质,范围为一到二;An为阴离子,特别适用于染色天然纤维、合成纤维形成材料和纤维素材料。这些染料可使棉花和纸张呈现各种稳定的黄色、红色和橙色。这些染料在纸张上的染色不易渗出,漂白时与次氯酸盐或氯漂白剂一起具有高度的色彩释放度。
-
PHOSPHONIUM COMPOUND, EPOXY RESIN COMPOSITION INCLUDING THE SAME AND SEMICONDUCTOR DEVICE PREPARED FROM THE SAME申请人:SAMSUNG SDI CO., LTD.公开号:US20160115184A1公开(公告)日:2016-04-28A phosphonium compound, an epoxy resin composition, a method of preparing a phosphonium compound, and a semiconductor device encapsulated with the epoxy resin composition, the compound being represented by Formula 1:
-
鏻化合物、制备其的方法、包括其的环氧树脂组合物以及由其制备的半导体装置
-
Substituted 1 and 2 naphthol mannich bases申请人:——公开号:US20040044061A1公开(公告)日:2004-03-04The invention relates to substituted 1 and 2 naphthol Mannich bases, a method for the production thereof, medicaments containing said compounds and the use of said compounds in the production of medicaments.该发明涉及取代的1和2-萘酚曼尼希碱,其生产方法,含有该化合物的药物以及该化合物在药物生产中的应用。
表征谱图
-
氢谱1HNMR
-
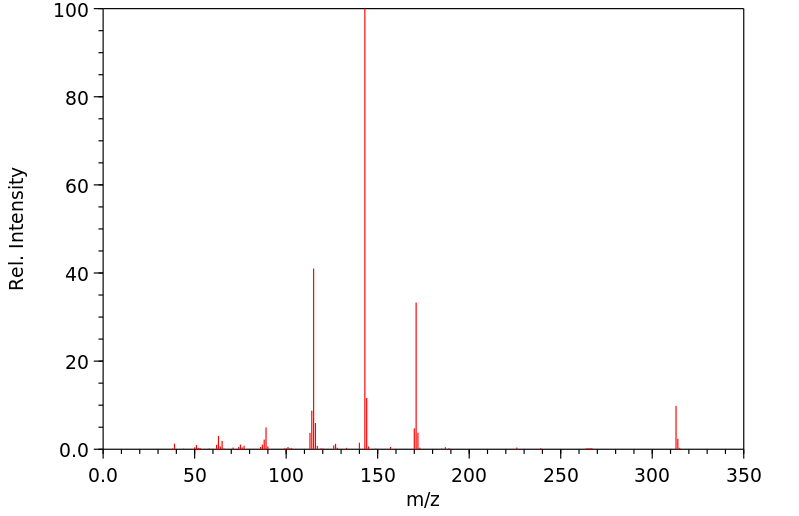
质谱MS
-
碳谱13CNMR
-
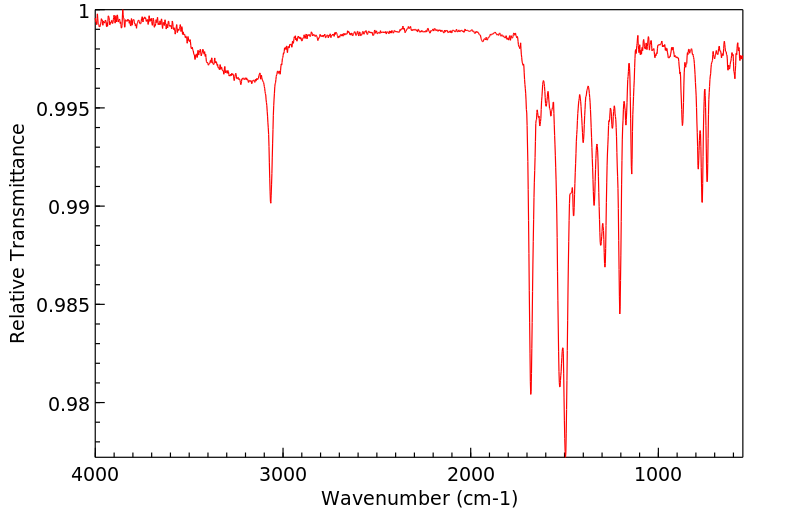
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮