7-氧杂-7H-苯并[de]蒽 | 200-23-7
中文名称
7-氧杂-7H-苯并[de]蒽
中文别名
7-氧杂-7H-苯并[DE]蒽
英文名称
benzo[kl]xanthene
英文别名
8-oxatetracyclo[7.7.1.02,7.013,17]heptadeca-1(16),2,4,6,9,11,13(17),14-octaene
CAS
200-23-7
化学式
C16H10O
mdl
——
分子量
218.255
InChiKey
QKOSFCWXOIAFTO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:109 °C
-
沸点:395 °C
-
密度:1.257±0.06 g/cm3(Predicted)
-
保留指数:360.96;360.96;361.38;361.6;359.85;359.77;361.38
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:17
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2932999099
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-萘-1-基苯酚 1-(2′-hydroxyphenyl)naphthalene 101277-90-1 C16H12O 220.271 1-(2-甲氧基苯基)萘 1-(2-methoxyphenyl)naphthalene 93321-11-0 C17H14O 234.298 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— benzoxantphos 261733-20-4 C40H28OP2 586.609
反应信息
-
作为反应物:描述:7-氧杂-7H-苯并[de]蒽 在 potassium permanganate 、 sodium dichromate 、 乙醇 、 sodium 、 溶剂黄146 作用下, 生成 1-carboxymethyl-9-xanthenone参考文献:名称:Kruber, Chemische Berichte, 1937, vol. 70, p. 1556,1563摘要:DOI:
-
作为产物:参考文献:名称:中间体醌甲基苯甲酸酯对苯酚的光环化和光加成反应摘要:制备了一系列的五种2-苯酚苯甲酰化衍生物,并对其光化学进行了研究。这些中的两个(3-苯基-2-萘酚10和1-苯基-2-萘酚11)是光惰性的。对于2-(1-萘基)苯酚(12)和1-(1-萘基)-2-萘酚(13),ESPT发生在萘环的2'-位或7'-位,得到进行反质子转移(RPT)或环电闭合以生成二氢苯并氧杂蒽的醌甲基化物(QMs)。检测了12和13的中间QM,并通过激光闪光光解进行了表征。对于2-(9-菲基)苯酚(14),ESPT发生在5'位以得到经过定量电环闭环的QM,以得到相应的苯并氧杂蒽,或在10'位发生以得到经历RPT的QM。如果溶液含有甲醇中,QM上ESPT产生至10'-位置14可以被捕获作为光加成产物。在这项工作中研究的化合物证明了ESPT之后产生的QM与芳族碳原子的三种可能反应:(1)反向质子转移(RPT)以再生起始原料;(2)加入羟基溶剂得到光致加成产物;(3)通过电环闭环得到苯并氧杂蒽衍生物。DOI:10.1021/acs.joc.5b01580
文献信息
-
Catalyst‐Free Synthesis of O‐Heteroacenes by Ladderization of Fluorinated Oligophenylenes作者:Mikhail Feofanov、Vladimir Akhmetov、Ryo Takayama、Konstantin AmsharovDOI:10.1002/anie.202007427日期:2021.3substitution even in the presence of strong electron‐donating groups and enables de‐tert‐butylation required for the complete annulation. Also demonstrated is the applicability of the method to introduce five‐, six‐, and seven‐membered rings containing oxygen, whereas multiple annulations also open up a short synthetic path to ladder‐type O‐heteroacenes and oligodibenzofurans.
-
Formation of dibenzofurans by flash vacuum pyrolysis of aryl 2-(allyloxy)benzoates and related reactions作者:Michael Black、J. I. G. Cadogan、Hamish McNabDOI:10.1039/c002480e日期:——Flash vacuum pyrolysis (FVP) of aryl 2-(allyloxy)benzoates 5 and of the corresponding aryl 2-(allylthio)benzoates 6 at 650 °C, gives dibenzofurans 19 and dibenzothiophenes 20, respectively. The mechanism involves generation of phenoxyl (or thiophenoxyl) radicals by homolysis of the O-allyl (or S-allyl) bond, followed by ipso attack at the ester group, loss of CO2 and cyclisation of the resulting aryl在650℃下,对 2-(烯丙氧基)苯甲酸芳基酯5和相应的2-(烯丙硫基)苯甲酸芳基酯6进行快速真空热解(FVP),分别得到二苯并呋喃19和二苯并噻吩20。该机制涉及产生苯氧基 (或者 噻吩氧基)通过O-烯丙基(或小号烯丙基)键,然后在酯基上进行ipso攻击,CO 2损失和所得芳基自由基环化。综合而言,该方法对p-取代的底物效果很好,可产生2-取代的二苯并呋喃19b-f(73-90%)和二苯并噻吩20b-c(90-94%)。在m-取代的底物的环化以及自由基与取代基和ipso-攻击的竞争性相互作用中,几乎没有选择性显示出o取代的底物的热解复杂化。相关自由基前体的FVP包括2-(烯丙氧基)苯基苯甲酸酯43没有产生二苯并呋喃,而2-(烯丙氧基-5-甲基)偶氮苯 44降低了产量。通过FVP不能获得咔唑2-(烯丙基氨基)苯甲酸4-甲基苯酯 42。
-
Palladium-Catalyzed Cross-Coupling of Benzyl Ketones and<i>α,β</i>-Unsaturated Carbonyl and Phenolic Compounds with<i>o</i>-Dibromobenzenes to Produce Cyclic Products作者:Yoshito Terao、Tetsuya Satoh、Masahiro Miura、Masakatsu NomuraDOI:10.1246/bcsj.72.2345日期:1999.10A number of carbonyl and phenolic compounds efficiently couple with o-dibromobenzenes in the presence of a palladium catalyst and a base to give the corresponding oxygen-containing heterocycles or carbocyclic compounds. Thus, from the reactions of benzyl phenyl ketones, 1-naphthols, and α,β-unsaturated aldehydes and ketones, benzofuran, benzopyran, benzocyclobutane, and indene derivatives, respectively, are produced selectively via the successive formation of C–C and C–O bonds or of two C–C bonds.
-
NMR and DFT studies on persistent carbocations derived from benzo[<i>kl</i> ]xanthene, dibenzo[<i>d</i> ,<i>d</i> ′]benzo[1,2-<i>b</i> :4,3-<i>b</i> ′]difuran, and dibenzo[<i>d</i> ,<i>d</i> ′]benzo[1,2-<i>b</i> :4,5-<i>b</i> ′]difuran in superacidic media作者:Takao Okazaki、Madoka Nakagawa、Takeshi Futemma、Toshikazu KitagawaDOI:10.1002/poc.3505日期:2016.2carbocations generated by the protonation of hetero‐polycyclic aromatic compounds with oxygen atom(s) were studied by experimental NMR and density function theory calculations. Benzo[kl]xanthene (1), dibenzo[d,d′]benzo[1,2‐b:4,3‐b′]difuran (2), and dibenzo[d,d′]benzo[1,2‐b:4,5‐b′]difuran (3) were synthesized by the annulation of arenediazonium salts. Compound 1 in FSO3H‐SbF5 (4:1)/SO2ClF and 3 in FSO3H‐SbF5 (1:1)/SO2ClF通过实验NMR和密度函数理论计算研究了杂多环芳族化合物与氧原子质子化产生的持久碳阳离子。苯并[ kl ]吨蒽(1),二苯并[ d,d ']苯并[1,2- b:4,3- b ']二呋喃(2)和二苯并[ d,d ']苯并[1,2- b:4,5- b ']二呋喃(3)是通过芳构氮鎓盐的环化反应合成的。FSO 3 H-SbF 5(4:1)/ SO 2 ClF和3中的化合物1在FSO 3 H-的SbF 5(1:1)/ SO 2的ClF电离以1AH +与质子化在C(4)和四氢-3aH- +与质子化在C(6),以及这些阳离子被成功通过NMR在低温下观察到。密度泛函理论计算表明,1aH +和3aH +是最稳定的质子化碳正离子,2应当在C(6)质子化下电离为2aH +。根据在变化13个c。化学位移(Δδ 13 C)中,正电荷被离域到用于萘单元1AH+,为一个苯并[ b, d ]呋喃单元2AH +,并进入一个苯并[
-
[EN] DIBENZOXANTHENE COMPOUND, ORGANIC LIGHT-EMITTING DEVICE, DISPLAY, IMAGE INFORMATION PROCESSOR, AND IMAGE-FORMING APPARATUS<br/>[FR] COMPOSÉ DE DIBENZOXANTHÈNE, DISPOSITIF ÉLECTROLUMINESCENT ORGANIQUE, AFFICHAGE, PROCESSEUR DE DONNÉES D'IMAGES, ET APPAREIL FORMANT DES IMAGES申请人:CANON KK公开号:WO2014007186A1公开(公告)日:2014-01-09Provided is a novel compound having a high lowest triplet excited level (T1 level), a narrow bandgap, and a shallow highest occupied molecular orbital (HOMO) level. A dibenzoxanthene compound is represented by formula [1] described in Claim 1. In formula [1], R1 to R7 are each independently selected from the group consisting of hydrogen, alkyl groups, aryl groups, heterocyclic groups, aryloxy groups, alkoxy groups, amino groups, silyl groups, and cyano groups.
表征谱图
-
氢谱1HNMR
-
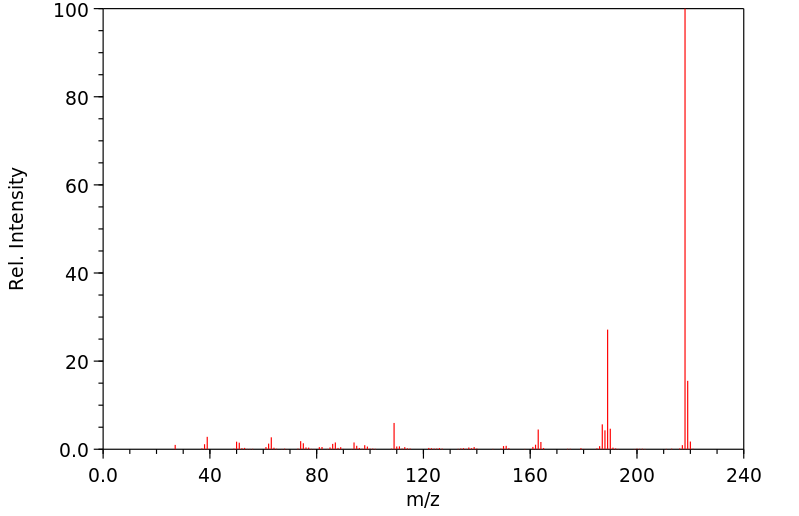
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-8-氟苯并二氢吡喃-4-胺
(2S)-6-氟-3,4-二氢-4-氧代-2H-1-苯并吡喃-2-甲酸
(2R)-6-氟-3,4-二氢-4-氧代-2H-1-苯并吡喃-2-甲酸
龙胆根素
龙胆SHAN酮
齿阿米素
黑色素-1
黄天精
麥角黃酮酸
鲍迪木醌
高邻苯二甲酸酐
高芒果苷
高氯酸罗丹明640
马佐卡林
香豆素-340
香豆素 339
食用色素红色105号
颜料红90:1铝色淀[CI45380:3]
颜料红172铝色淀[CI45430:1]
雏菊叶龙胆酮
降阿赛里奥; 1,3,6,7-四羟基氧杂蒽酮
阿米醇
阿米凯林
阿米凯林
阿扎那托
阿巴哌酮
阿尼地坦
阿尼地坦
阿匹氯铵
锌离子荧光探针-4
锆(2+)二[2-(2,4,5,7-四溴-3,6-二羟基氧杂蒽-9-基)-3,4,5,6-四氯苯甲酸酯]
铁力木呫吨酮-B
铁力木吨酮A
钠6'-羟基-5-[2-[4-(2-甲基-3-氧代-7H-咪唑并[1,2-d]吡嗪-6-基)苯氧基]乙基硫代氨基甲酰氨基]-3-氧代螺[2-苯并呋喃-1,9'-氧杂蒽]-3'-醇
钠4-{[6'-(二乙基氨基)-3'-羟基-3-氧代-3H-螺[2-苯并呋喃-1,9'-氧杂蒽]-2'-基]偶氮}-3-羟基-1-萘磺酸酯
钠2-(2,7-二氯-9H-氧杂蒽-9-基)苯甲酸酯
钙黄绿素乙酰甲酯
钙荧光探针Fluo-8,AM
钙荧光探针Fluo-4,AM
钙离子荧光探针
钙柑子
采木(HAEMATOXYLONCAMPECHIANUM)木质提取物
酸性媒介桃红3BM
邻苯三酚红
远志山酮III
转移核糖核酸(面包酵母)
赤藓红B异硫氰酸酯异构体II
赤藓红
诺大麻
西伯尔链接剂