9,10-二(苯基甲基)-蒽 | 3613-42-1
中文名称
9,10-二(苯基甲基)-蒽
中文别名
——
英文名称
9,10-dibenzylanthracene
英文别名
9,10-dibenzyl-anthracene;9,10-Dibenzyl-anthracen;9,10-bis(phenylmethyl)-anthracene;9.10-Dibenzyl-anthracen;9,10-Dibenzylanthracen
CAS
3613-42-1
化学式
C28H22
mdl
——
分子量
358.483
InChiKey
FMVRRCDBALUUBI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:246.5°C
-
沸点:425.89°C (rough estimate)
-
密度:1.1787
计算性质
-
辛醇/水分配系数(LogP):8.3
-
重原子数:28
-
可旋转键数:4
-
环数:5.0
-
sp3杂化的碳原子比例:0.07
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902909090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— ethyl-[(10-benzyl-[9]anthryl)-phenyl-methyl]-ether 408317-56-6 C30H26O 402.536
反应信息
-
作为反应物:描述:参考文献:名称:Plastic Scintillators. III. The Synthesis of Some Anthracene Derivatives as Wavelength Shifters in Plastic Scintillators摘要:本文合成了 15 种蒽衍生物,并测量了它们的吸收和荧光光谱。本文将对这些光谱进行讨论。根据光谱结果,本文合成的一些蒽衍生物比以前文献中描述的化合物具有更好的波长偏移性。DOI:10.1246/bcsj.39.1551
-
作为产物:描述:参考文献:名称:Mixed sulfonic-carboxylic anhydrides. III. Reactions with aromatic ethers and aromatic hydrocarbons摘要:DOI:10.1021/jo00803a011
文献信息
-
FACILE GENERATION OF ALKYL RADICALS BY THE PALLADIUM ON CARBON CATALYZED THERMOLYSIS OF ALKYLMERCURIALS作者:Michiko Iwamura、Shohaku Futibe、Tetuo Matukura、Masahide SanoDOI:10.1246/cl.1983.1623日期:1983.10.5Dibenzylmercury was completely decomposed within 20 min in refluxing xylene in the presence of a catalytic amount of 5% palladium on carbon, giving mercury and dibenzyl. The generation of benzyl radicals was confirmed by the formation of 1,3-adduct to α-phenyl-N-benzylnitrone and 9,10-adduct to anthracene. Other alkylmercurials showed a similar but less satisfactory result.
-
The unusual behaviour of nitro-substituted radical σ-complexes. The reactions of alkyl radicals with 9-nitroanthracene作者:Lorenzo Testaferri、Marco Tingoli、Marcello TieccoDOI:10.1039/p29830000543日期:——Alkyl radicals react with 9-nitroanthracene to give the nitro-substituted σ-complex intermediate (III) derived from addition at the 10-position. This radical does not rearomatize, but gives rise to rearrangement of the nitro-group which eventually leads to the formation of alkylanthrones. However, in the presence of stabilized radicals, intermediate (III) is trapped to give products of addition at
-
Low temperature Kumada–Corriu cross-coupling of polychlorinated acene derivatives and a synthesis of sterically demanding acenes作者:Elisey Yagodkin、Christopher J. DouglasDOI:10.1016/j.tetlet.2010.03.121日期:2010.6linear acenes for organic materials applications. Treatment of polychlorinated acenes with the PEPPSI-IPr catalyst and MeMgBr undergo 6–8 concurrent coupling reactions to yield products such as octamethylnaphthalene, which is distorted out of planarity due to the steric interaction between the methyl groups. More sterically demanding Grignard reagents such as PhMgBr coupled cleanly with 9,10-dichloroanthracene
-
Barnett; Cook, Journal of the Chemical Society, 1928, p. 572作者:Barnett、CookDOI:——日期:——
-
Lippmann; Fritsch, Monatshefte fur Chemie, 1904, vol. 25, p. 795作者:Lippmann、FritschDOI:——日期:——
表征谱图
-
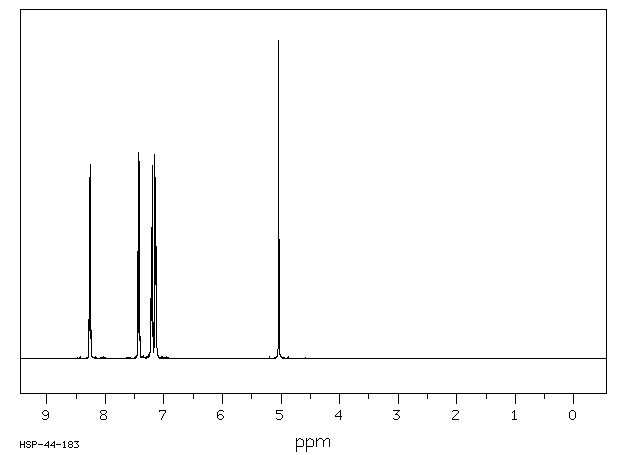
氢谱1HNMR
-
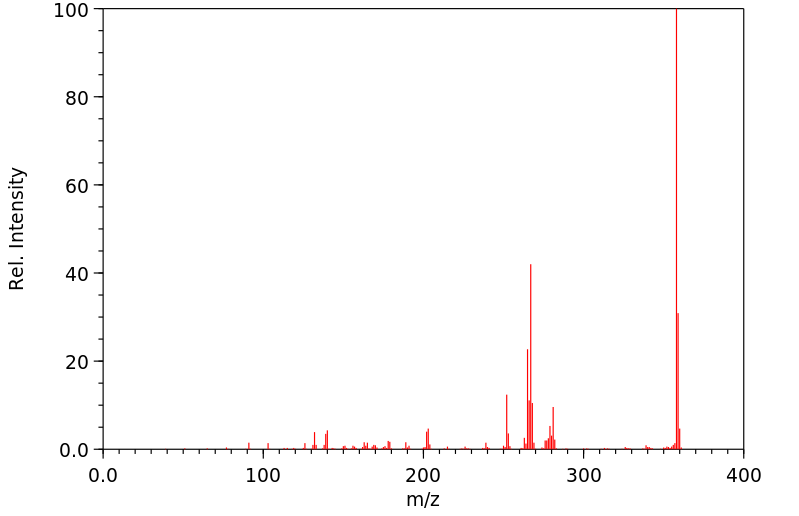
质谱MS
-
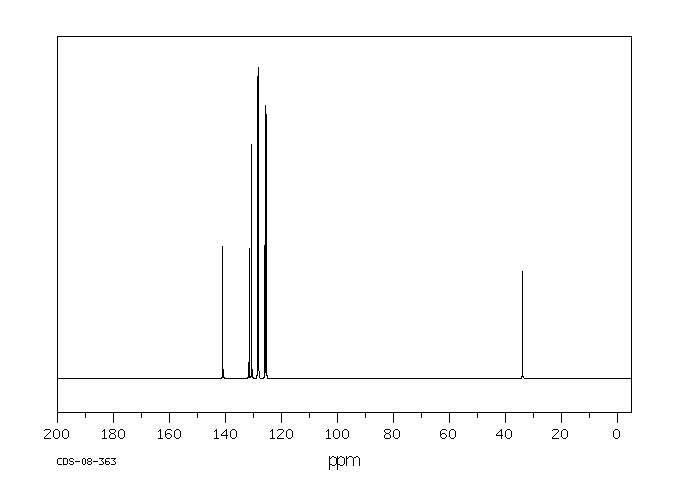
碳谱13CNMR
-
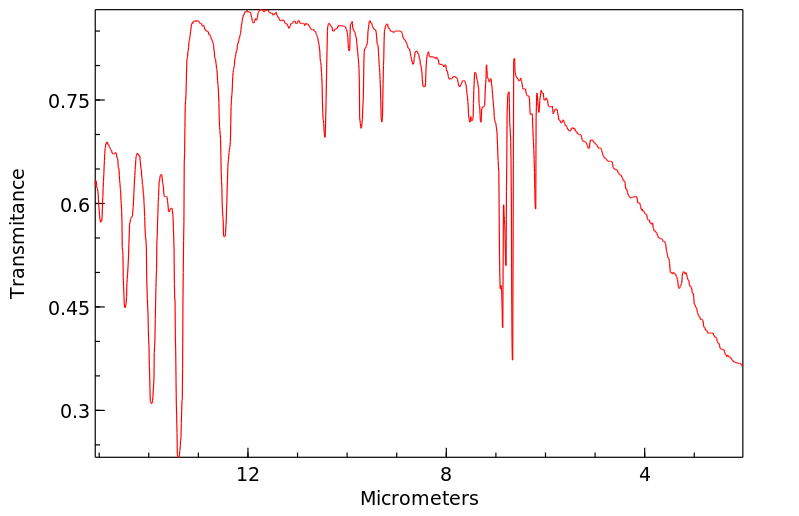
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62