9-苯氧基蒽 | 74067-56-4
中文名称
9-苯氧基蒽
中文别名
——
英文名称
phenoxy-9 anthracene
英文别名
9-Phenoxyanthracene;9-Phenyloxy-anthracen;9-Phenoxy-anthracen
CAS
74067-56-4
化学式
C20H14O
mdl
——
分子量
270.331
InChiKey
STICXSFPZUNCRS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:132-133 °C
-
沸点:449.9±14.0 °C(Predicted)
-
密度:1.184±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):6
-
重原子数:21
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 9-溴-10-苯氧基蒽 bromo-9 phenoxy-10 anthracene 23674-00-2 C20H13BrO 349.227 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 9-溴-10-苯氧基蒽 bromo-9 phenoxy-10 anthracene 23674-00-2 C20H13BrO 349.227
反应信息
-
作为反应物:描述:参考文献:名称:卤化铜与有机化合物的反应-IV:9-烷氧基-和9-酰氧基蒽的反应摘要:9-烷氧基和9-酰氧基蒽与溴化铜和氯化铜反应,得到9-烷氧基(或酰氧基)10-卤代蒽和/或联蒽9-基。前一种产物的形成是通过卤化铜的卤素的配体转移而进行的,而苯硫隆-9-基则是由9-蒽氧基自由基的二聚作用产生的。它们在取代的蒽和卤化铜之间以4中心的过渡态形成。两种产物的比例和相对反应速率根据上述方法来解释。还研究了9-甲基硫代蒽与卤化铜的反应。DOI:10.1016/s0040-4020(01)82884-2
-
作为产物:描述:参考文献:名称:竞争进入替代亲核试剂和还原溴代9蒽酚,阴离子酚盐和甲醇盐的应用摘要:用DMF中的苯酚钾处理α位不带氢的内取代的9-溴蒽在亲核取代和还原脱卤之间引起竞争。带有电子吸引基团1c(Br),1e(CN)和1f(NO 2)的衍生物基本上通过取代反应生成酚醚2,而与未活化的溴化物1a(H),1b(C 6 H 5))和1d(OC 6 H 5),主要反应是还原为蒽3 这应该是由电子转移引起的。DOI:10.1016/0040-4020(82)80052-5
文献信息
-
Restricted Rotation Involving the Tetrahedral Carbon. LVII. Stereodynamics of 9-(2-Alkylphenoxy)-1,4-dimethyltriptycenes作者:Gaku Yamamoto、Michinori OkiDOI:10.1246/bcsj.58.1953日期:1985.71,4-Dimethyl-9-phenoxytriptycene and its derivatives carrying a methyl, isopropyl, or t-butyl group in the o-position of the phenoxyl moiety were synthesized and their dynamic NMR behavior was studied. These molecules are considered to constitute a bevel gear system with a two-toothed and a three-toothed wheels and the dynamic NMR behavior is best explained in terms of gear rotation. The o-isopropyl derivative, for example, shows the energy barriers of 17.2 and 10.8 kcal mol−1 for the ap\ightleftharpoons±sc and +sc\ightleftharpoons−sc gearing processes, respectively. The population of the ±sc rotamer increases with the bulkiness of the o-alkyl group: The steric congestion among the o-alkyl group, the oxygen atom, and the peri-methyl group is severer in ap than in ±sc. Results of molecular mechanics calculations on these molecules are discussed.
-
OBSERVATION OF RESTRICTED ROTATION ABOUT C–O BONDS BY NMR SPECTROSCOPY IN 9-(2-ALKYLPHENOXY)TRIPTYCENES, A GEAR ROTATION SYSTEM作者:Gaku Yamamoto、Michinori OkiDOI:10.1246/cl.1984.97日期:1984.1.54-dimethyltriptycenes revealed that, when the o-alkyl group is methyl or isopropyl, two conformers, ap and ±sc, are separately observed at room temperature and that the interconversion between them occurs by gear rotation with an energy barrier of ca. 18 kcal mol−19-(2-烷基苯氧基)-1,4-二甲基三萜烯的动态核磁共振研究表明,当邻烷基为甲基或异丙基时,在室温下分别观察到两个构象异构体 ap 和 ±sc,并且它们通过具有约能量势垒的齿轮旋转发生。18 kcal mol−1
-
Theilacker,W. et al., Chemische Berichte, 1960, vol. 93, p. 1658 - 1681作者:Theilacker,W. et al.DOI:——日期:——
-
Dynamic stereochemistry of molecular gears, 9-benzyltriptycene and 9-phenoxytriptycene. studied by 13C dynamic NMR spectroscopy and molecular mechanics calculations作者:Gaku YamamotoDOI:10.1016/s0040-4020(01)88370-8日期:1990.1
-
RIGAUDY, J.;SEULEIMAN, A. M.;CUONG, NGUYEN, KIM, TETRAHEDRON, 1982, 38, N 21, 3157-3161作者:RIGAUDY, J.、SEULEIMAN, A. M.、CUONG, NGUYEN, KIMDOI:——日期:——
表征谱图
-
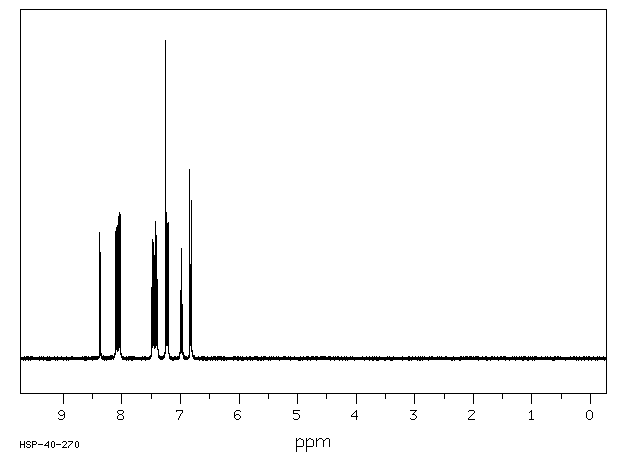
氢谱1HNMR
-
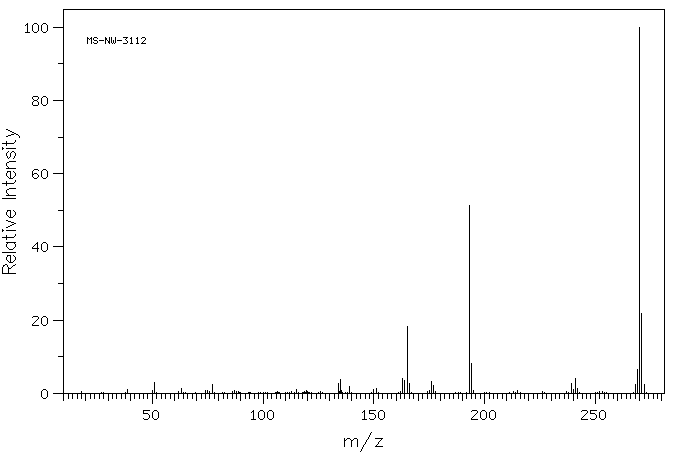
质谱MS
-
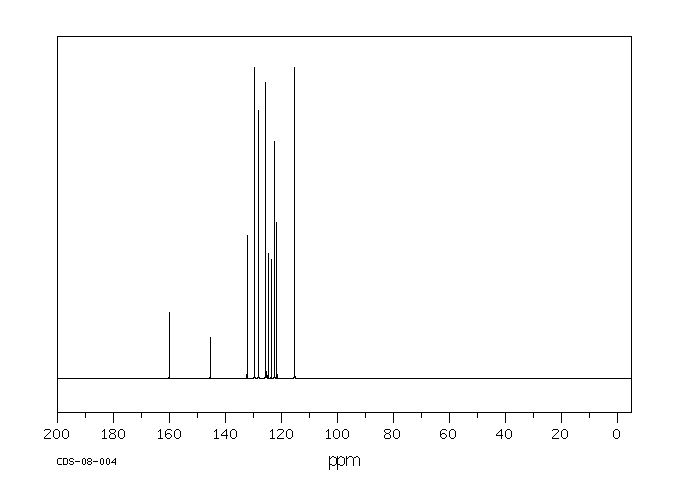
碳谱13CNMR
-
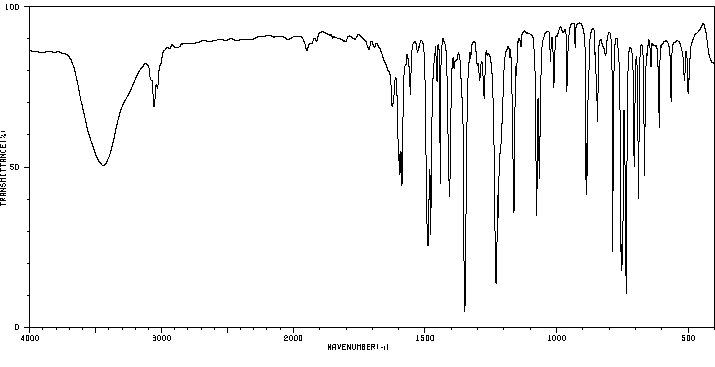
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62