苯并二氢呋喃二酮 | 4732-72-3
中文名称
苯并二氢呋喃二酮
中文别名
苯并呋喃-2,3-二酮
英文名称
coumarandione
英文别名
benzofuran-2,3-dione;Cumarandion;Cumaran-dion-(2,3);Coumaran-2,3-dion;3-oxobenzofuran-2-one;2,3-Benzofurandione;1-benzofuran-2,3-dione
CAS
4732-72-3
化学式
C8H4O3
mdl
MFCD09035228
分子量
148.118
InChiKey
UUISWLJHAJBRAA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:120℃
-
沸点:228.68°C (rough estimate)
-
密度:1.444
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— (2-hydroxyphenyl)oxoacetic acid 17392-16-4 C8H6O4 166.133 —— 2-(hydroxyimino)benzofuran-3(2H)-one —— C8H5NO3 163.133 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (2-hydroxyphenyl)oxoacetic acid 17392-16-4 C8H6O4 166.133 3-羟基-1-苯并呋喃-2(3H)-酮 3-hydroxy-3H-benzofuran-2-one 22303-62-4 C8H6O3 150.134 —— methyl 2-(2-methoxymethoxybenzyloxy)phenyl-glyoxylate 299957-44-1 C18H18O6 330.337 —— 2-Ethoxycarbonylmethylene-3(2H)-benzofuranone 130900-88-8 C12H10O4 218.209
反应信息
-
作为反应物:描述:参考文献:名称:一种用于医院护理杀菌消毒的噁唑类化合物及其制备方法和应用摘要:本发明公开了一种用于医院护理杀菌消毒的噁唑类化合物及其制备方法和应用,属于抗菌药物合成技术领域。本发明的技术方案要点为:该噁唑类化合物的结构为:其中R为烷基,烯烃基团,芳环等取代基团。本发明通过水解苯并二氢呋喃酮得到酸,再和胺基炔缩合后利用酰胺基团的烯醇式结构与乙酸酯缩合得到噁唑基团,再利用端基炔和胺基成环得到了六元环结构,最后引入带有羧酸的结构得到了多种结构新颖的化合物,并具有一定的抑菌活性。公开号:CN112898317A
-
作为产物:参考文献:名称:双杂环螺3(2 H)-呋喃酮的偶然合成摘要:(Ž)烯醇三氟甲磺酸酯6,11B - d,(ê)烯醇三氟甲磺酸11E,和三氟甲磺酸酚11A,从β酮酯或2-烷氧羰基的酚衍生的,分别与反应Ñ -Boc 2- lithiopyrrolidine(图5a),Ñ -Boc N-甲基氨基甲基锂(5b)或2-硫代-1,3-二硫杂环丁烷(14)以适中至良好的收率(38–81%)提供3(2 H)-呋喃酮。产品和碳负离子试剂研究表明3(2 H呋喃酮是在一系列反应中形成的,包括亲核酰基取代,烯醇化物形成,三氟甲基转移,亚胺或sulf离子形成,以及随后的闭环反应,形成3(2 H)-呋喃酮。使用2-硫代-1,3-二硫杂环丁烷可得到环状α-酮基-S,S,O-原酸酯,其中官能团可以选择性地用于合成应用。DOI:10.1021/acs.joc.5b02350
文献信息
-
一种用于杀菌消毒的咪唑类化合物及其制备方法和应用
-
[EN] BENZOFURAN-4,5-DIONES AS SELECTIVE PEPTIDE DEFORMYLASE INHIBITORS<br/>[FR] BENZOFURANE-4-5-DIONES CONSTITUANT DES INHIBITEURS SÉLECTIFS DE LA DÉFORMYLASE DE PEPTIDE申请人:SLOAN KETTERING INST CANCER公开号:WO2010129049A1公开(公告)日:2010-11-11The instant invention provides novel benzofuran-4,5-diones and pharmaceutical compositions thereof useful for inhibiting PDF and for treating proliferative and infectious diseases. Compounds may be selective for eukaryotic (e.g., human) PDF or prokaryotic PDF.
-
Synthesis and Antitumor Properties of <i>N</i>-[2-(Dimethylamino)ethyl]carboxamide Derivatives of Fused Tetracyclic Quinolines and Quinoxalines: A New Class of Putative Topoisomerase Inhibitors作者:Leslie W. Deady、Anthony J. Kaye、Graeme J. Finlay、Bruce C. Baguley、William A. DennyDOI:10.1021/jm970044r日期:1997.6.1does not result primarily from inhibition of topo II. The quinoxaline analogues had more varied IC50 values, being on average less cytotoxic than the quinoline derivatives, but appeared to have a similar mode of action. Overall, this new class of compounds appear to be mixed topo I/II inhibitors, up to 3-fold more cytotoxic than DACA in the human leukemia cell lines studied, with in vivo activity in制备了一系列四环喹啉和喹喔啉羧酰胺,并在一系列鼠类人肿瘤细胞系中评估了它们的细胞毒性。多数喹啉衍生物是通过适应Pfitzinger合成,随后进行热脱羧并使用氯甲酸异丁酯通过混合酸酐法与N,N-二甲基乙二胺偶联而制备的。喹啉类似物显示出与已知的三环a啶-4-羧酰胺混合的topoI / II抑制剂DACA相似的细胞毒性,其中噻吩和茚满类似物最具活性。他们显示出对Jurkat人白血病topo II耐药株JLA和JLC的效力几乎没有降低,表明它们的细胞毒性并非主要是由于对topo II的抑制所致。喹喔啉类似物的IC50值变化更大,与喹啉衍生物相比,其平均细胞毒性要低,但似乎具有相似的作用方式。总的来说,这类新化合物似乎是混合的topo I / II抑制剂,在研究的人类白血病细胞系中的细胞毒性比DACA高三倍,在结肠38的体内活性与DACA和阿霉素相当。
-
PROCESS FOR PREPARING DIARYL OXALATE申请人:Tanaka Shuji公开号:US20120296063A1公开(公告)日:2012-11-22Disclosed is a process for preparing a diaryl oxalate which comprises the step of transesterifying a dialkyl oxalate or/and an alkylaryl oxalate with an aryl alcohol in the presence of a tetra(aryloxy)titanium as a catalyst, wherein the tetra(aryloxy)titanium is fed into a reaction system of the transesterification as an aryl alcohol solution of the tetra(aryloxy)titanium which is prepared by reacting a tetraalkoxy titanium and an excess amount of the aryl alcohol and removing a by-producing alkyl alcohol.
-
[EN] PROCESS FOR PREPARING (E)-(5,6-DIHYDRO-1,4,2-DIOXAZINE-3-YL) (2-HYDROXYPHENYL) METHANONE O-METHYL OXIME<br/>[FR] PROCÉDÉ DE PRÉPARATION DE O-MÉTHYL OXIME DE (E)-(5,6-DIHYDRO-1,4,2-DIOXAZIN-3-YL) (2-HYDROXYPHÉNYL)MÉTHANONE申请人:ARYSTA LIFESCIENCE CORP公开号:WO2016193822A1公开(公告)日:2016-12-08A process for preparing (E)-(5,6-dihydro-l,4,2-dioxazin-3-yl)(2- hydroxyphenyl)methanone O-methyl oxime is described which includes: (i) reacting benzofuran-3(2H)-one O-methyl oxime (1) with at least one nitrite selected from n-butyl nitrite and tert-butyl nitrite, in the presence of a metal alkoxide to form (2Z,32)-2,3-benzofuran-dione O3-methyl dioxime (2) as the predominant isomer; (ii) reacting the (2Z,3Z)-2,3-benzofuran-dione O -methyl dioxime (2) with 2- haloethanol to form (2Z,3Z)-benzofuran-2,3-dione 02-(2-hydroxyethyl) O3 -methyl dioxime (3); and (iii) reacting the (2Z,3Z)-benzofuran-2,3-dione 02-(2-hydroxyethyl) & -methyl dioxime (3) with an acid to form (E)-(5,6-dihydro-l,4,2-dioxazin-3-yl)(2- hydroxyphenyl)methanone (9-methyl oxime (4);描述了一种制备(E)-(5,6-二氢-1,4,2-二噁嗪-3-基)(2-羟基苯基)甲酮O-甲基肟的方法,包括:(i)将苯并呋喃-3(2H)-酮O-甲基肟(1)与n-丁基亚硝酸酯和叔丁基亚硝酸酯中至少一种进行反应,在金属烷氧化物存在下形成(2Z,3Z)-2,3-苯并呋喃二酮O-3-甲基二肟(2)作为主要异构体;(ii)将(2Z,3Z)-2,3-苯并呋喃二酮O-甲基二肟(2)与2-卤乙醇反应,形成(2Z,3Z)-苯并呋喃-2,3-二酮O2-(2-羟乙基)O3-甲基二肟(3);(iii)将(2Z,3Z)-苯并呋喃-2,3-二酮O2-(2-羟乙基)O-甲基二肟(3)与酸反应,形成(E)-(5,6-二氢-1,4,2-二噁嗪-3-基)(2-羟基苯基)甲酮O-甲基肟(4)。
表征谱图
-
氢谱1HNMR
-
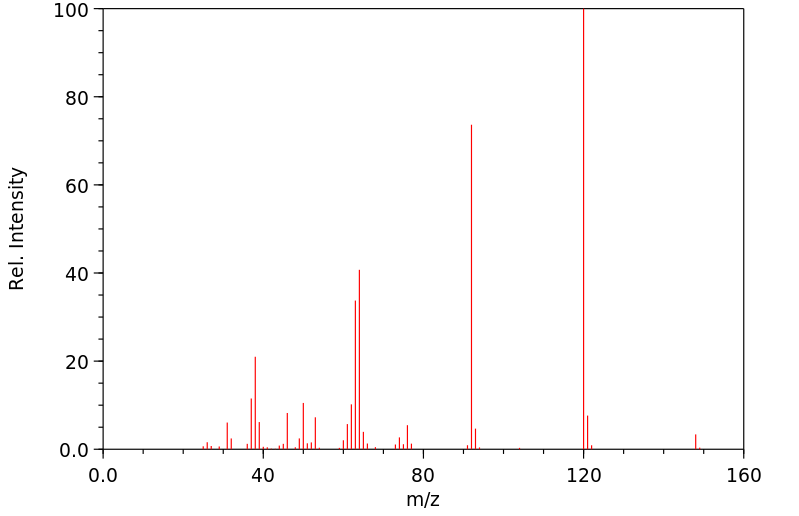
质谱MS
-
碳谱13CNMR
-
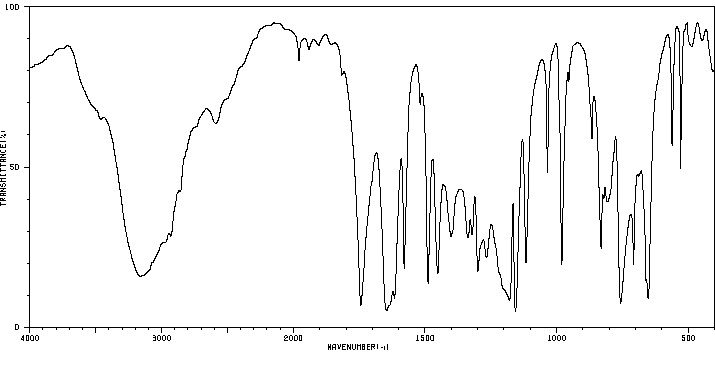
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
()-2-(5-甲基-2-氧代苯并呋喃-3(2)-亚乙基)乙酸乙酯
顺式-1-((2-(5-氯-2-苯并呋喃基)-4-甲基-1,3-二氧戊环-2-基)甲基)-1H-1,2,4-三唑
顺式-1-((2-(5,7-二氯-2-苯并呋喃基)-4-乙基-1,3-二氧戊环-2-基)甲基)-1H-咪唑
顺式-1-((2-(2-苯并呋喃基)-4-乙基-1,3-二氧戊环-2-基)甲基)-1H-1,2,4-三唑
霉酚酸酯杂质B
雷美替胺杂质3
雷美替胺杂质22
雷美替胺杂质
间甲酚紫
间甲基苯基(苯并呋喃-2-基)甲醇
长管假茉莉素C
钠1,4-二[(2-乙基己基)氧基]-1,4-二氧代-2-丁烷磺酸酯-3,3-二(4-羟基苯基)-2-苯并呋喃-1(3H)-酮(1:1:1)
金霉素
酪氨酸,b-羰基-
酞酸酐-d4
酚酞二丁酸酯
酚酞
酚红钠
酚红
邻苯二甲酸酐与马来酸酐,甘氨酰蜡素和二乙二醇的聚合物
邻苯二甲酸酐与己二醇的聚合物
邻苯二甲酸酐与三甘醇异壬醇的聚合物
邻苯二甲酸酐与2-乙基-2-羟甲基-1,3-丙二醇和2,5-呋喃二酮的聚合物
邻苯二甲酸酐与2-乙基-2-羟甲基-1,3-丙二醇、2,5-呋喃二酮和2-乙基己酸苯甲酸酯的聚合物
邻苯二甲酸酐-13C6
邻苯二甲酸酐-4-硼酸频哪醇酯
邻苯二甲酸酐,马来酸,二乙二醇,新戊二醇聚合物
邻甲酚酞二庚酸酯
邻甲酚酞二己酸酯
邻甲酚酞
贝康唑
表灰黄霉素
螺佐呋酮
螺[苯并呋喃-3(2H),4-哌啶]
螺[异苯并呋喃-1(3H),4’-哌啶]-3-酮
螺[异苯并呋喃-1(3H),4'-哌啶]-3-酮盐酸盐
螺[异苯并呋喃-1(3H),3’-吡咯烷]-3-酮
螺[1-苯并呋喃-2,1'-环丙烷]-3-酮
薄荷内酯
萘并[2,3-b]呋喃-8(4H)-酮,4a,5,6,7,8a,9-六氢-,顺-
莫罗卡尼
荨麻叶泽兰酮
荧光胺
苯酞-3-乙酸
苯酚,2-[3-(2-苯并呋喃基)-5,6-二氢-1,2,4-三唑并[3,4-b][1,3,4]噻二唑-6-基]-
苯酐二乙二醇共聚物
苯酐
苯甲酸,2-[(1,3-二羰基丁基)氨基]-,甲基酯
苯甲酸,2,2-二(羟甲基)丙烷-1,3-二醇,异苯并呋喃-1,3-二酮
苯甲酰氯化,3-甲氧基-4-甲基-