N,N-二甲基二硫代氨基甲酸二甲铵盐 | 598-64-1
中文名称
N,N-二甲基二硫代氨基甲酸二甲铵盐
中文别名
二甲氨基二甲基二硫代氨基甲酸酯;二甲基二硫代氨基甲酸二甲基铵盐
英文名称
dimethylammonium dimethyldithiocarbamate
英文别名
N,N-Dimethylammonium-N,N-dimethyldithiocarbamat;Dimethylamine dimethyldithiocarbamate;dimethylcarbamodithioic acid;N-methylmethanamine
CAS
598-64-1
化学式
C2H8N*C3H6NS2
mdl
——
分子量
166.312
InChiKey
UVOFGKIRTCCNKG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:132°C
-
密度:1.117 (estimate)
-
稳定性/保质期:
性质与稳定性:在常温常压下,该物质不会分解。
计算性质
-
辛醇/水分配系数(LogP):-0.81
-
重原子数:9
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:52.9
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险类别码:R36/37/38
-
海关编码:2930909090
-
储存条件:贮存: 将密器密封后,存放于密封的主要容器中,并置于阴凉、干燥处。
SDS
二甲基二硫代氨基甲酸二甲基铵盐 修改号码:5
模块 1. 化学品
产品名称: Dimethylammonium Dimethyldithiocarbamate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 二甲基二硫代氨基甲酸二甲基铵盐
百分比: >95.0%(T)
CAS编码: 598-64-1
俗名: Dimethyldithiocarbamic Acid Dimethylammonium Salt
二甲基二硫代氨基甲酸二甲基铵盐 修改号码:5
模块 3. 成分/组成信息
分子式: C5H14N2S2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体-粉末
二甲基二硫代氨基甲酸二甲基铵盐 修改号码:5
模块 9. 理化特性
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点:
132°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: FA0250000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
二甲基二硫代氨基甲酸二甲基铵盐 修改号码:5
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Dimethylammonium Dimethyldithiocarbamate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 二甲基二硫代氨基甲酸二甲基铵盐
百分比: >95.0%(T)
CAS编码: 598-64-1
俗名: Dimethyldithiocarbamic Acid Dimethylammonium Salt
二甲基二硫代氨基甲酸二甲基铵盐 修改号码:5
模块 3. 成分/组成信息
分子式: C5H14N2S2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体-粉末
二甲基二硫代氨基甲酸二甲基铵盐 修改号码:5
模块 9. 理化特性
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点:
132°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: FA0250000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
二甲基二硫代氨基甲酸二甲基铵盐 修改号码:5
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
制备方法
用途:用于有机物合成
用途简介用途:用于有机物合成
反应信息
-
作为反应物:描述:N,N-二甲基二硫代氨基甲酸二甲铵盐 以 甲醇 为溶剂, 反应 4.0h, 生成 3-[2-(dimethyl-thiocarbamoylsulfanyl)-ethyl]-4-methyl-2-methylsulfanyl-thiazolium; iodide参考文献:名称:Studies of heterocyclic compounds. VI. The reactions of 5,6-dihydrothiazolo(2,3-b)thiazolium salts with O- and S-nucleophiles.摘要:5, 6-二氢噻唑并[2, 3-b]噻唑鎓盐(1)与氢氧根离子反应得到3-(2-巯基乙基)-4-噻唑啉-2-酮(6)的二硫化物。 1与硫氢离子反应得到3-(2-巯基乙基)-4-噻唑啉-2-硫酮(7)和/或其二硫化物(8),与N,N-二甲基二硫代氨基甲酸根离子反应得到3-(N,N-)二甲基-二硫代氨基甲酰乙基)-4-噻唑啉-2-硫酮(12),而1b与苯硫酚根离子的反应得到3-(2-苯硫基乙基)-4-噻唑啉-2-硫酮(18b)、噻唑(21)、6b、苯基2-[2-(4-苯基噻唑啉-2-硫磷-3-基)乙硫基]乙基二硫醚(22)和苯基2-苯硫基乙基二硫醚(23)。讨论了这些产物形成的简要反应机理。 1的反应被认为是由亲核试剂攻击极化的>C=N<键形成加合物并通过AE机制进行的。反应的消除阶段取决于试剂的碱性、极化性和其他性质,以诱导 S7-C7a 键的裂解、S7-C7a 和 N-C5 键的裂解,或试剂对C5 或 S7。DOI:10.1248/cpb.23.3243
-
作为产物:描述:参考文献:名称:Utilization of carbon disulfide as a powerful building block for the synthesis of 2-aminobenzoxazoles摘要:此协议描述了一条新颖、温和且便捷的途径,能够在高产率下制备2-氨基苯并噁唑,标志着向环境友好型策略迈出了重要的一步。脂肪族胺与二硫化碳反应生成中间体二硫代氨基甲酸盐(DTC),在2-氨基苯酚的存在下,这些中间体随后经历连续的分子间亲核攻击和脱硫反应,在3小时内即可生成2-氨基苯并噁唑。DOI:10.1039/c3ra41189c
-
作为试剂:描述:参考文献:名称:v.Braun; Kaiser, Chemische Berichte, 1923, vol. 56, p. 550摘要:DOI:
文献信息
-
Hochdruckversuche: Umsetzungen von kohlenstoffdisulfid mit aminen, amiden und olefinen—V作者:J. Petermann、H. PlieningerDOI:10.1016/0040-4020(75)85058-7日期:1975.1Suitable tertiary amines react with CS2, 100°C, 104 atm to give N,N-disubstituted thioformamides. Salts of dialkyldithiocarbamic acids decompose at the same conditions into dialkylthioformamide and sulphur. After addition of cyclohexene, mainly N,N-dialkyldithiocarbamic acid cyclohexyl ester has been obtained. Carbon disulfide and sulphur add to olefins yielding trithiocarbonates. Dimethyl formamide
-
Thiocarbamyolation of amine-containing compounds作者:Luu Van BoiDOI:10.1007/bf02498275日期:1999.12secondary aliphatic amines with tetramethylthiuram disulfide in various solvents at different temperatures was studied. At 110°C, the reactions with primary amines afforded mixed,N,N-dimethyl-N′-alkyl(cycloalkyl)thioureas and symmetricalN,N′-dialkyl(cycloalkyl)thioureas as the final products, while the reactions with secondary amines gave mixtures of dithiocarbamate salts with “symmetrical” derivatives
-
Thiocarbamoylation of amine-containing compounds作者:Luu Van BoiDOI:10.1007/bf02494685日期:2000.2into the final products occur,viz., (1) the reactions of CS2 with primary amines on heating (70–110 °C) yield mixed and symmetrical thioureas and the reactions of CS2 with secondary amines give symmetrical dithiocarbamates, and (2) insertion of CS2 intoS-(thiocarbamoyl)thiohydroxylamines affords thiuram disulfides. Thiuram disulfides formed from primary amines decompose to give isothiocyanates, which
-
Cu-mediated one-pot three-component synthesis of 3-N-substituted 1,4,2-benzodithiazine 1,1-dioxide derivatives作者:Wei Dong、Zemei Ge、Xin Wang、Ridong Li、Runtao LiDOI:10.1016/j.tet.2020.131354日期:2020.7A novel and efficient copper-catalyzed one-pot procedure for the synthesis of 3-N-substituted 1,4,2-benzodithiazine 1,1-dioxide derivatives from 2-halobenzenesulfonamides, amines and CS2 is described. The reaction proceeds through Ullmann-type S-arylation, intramolecular addition of NH2 with CS and dehydrosulfide, which provides a new and useful strategy for construction of cyclic aromatic sulfonamides
-
A catalyst-free 1,4-Michael-type reaction of in situ generated ortho-quinone methides (o-QMs) with dithiocarbamic acid salts in water作者:Fezzeh Aryanasab、Meisam ShabanianDOI:10.1007/s13738-019-01644-z日期:2019.8catalyst-free conjugate addition of dithiocarbamic acid salts to in situ generated ortho-quinone methides (o-QMs) was investigated for the first time. Several dithiocarbamate derivatives of 4-hydroxycoumarine, 4-hydroxypyrone and 2-naphthol were synthesized in moderate-to-good yields in water at room temperature. Graphical abstract Catalyst-free addition of dithiocarbamic acid salts to in situ generated o-QMs
表征谱图
-
氢谱1HNMR
-
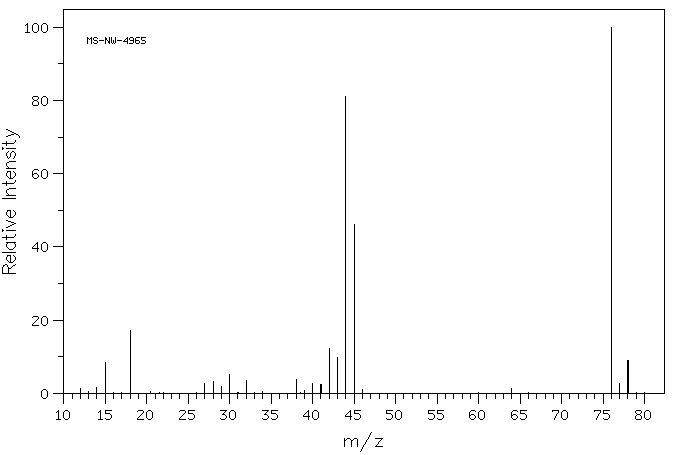
质谱MS
-
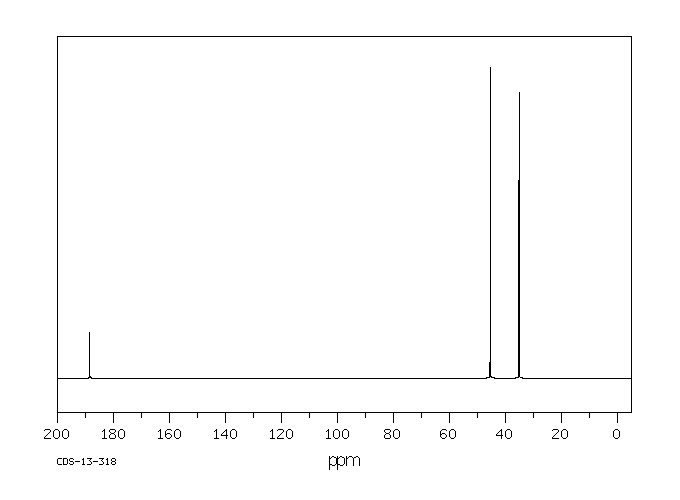
碳谱13CNMR
-
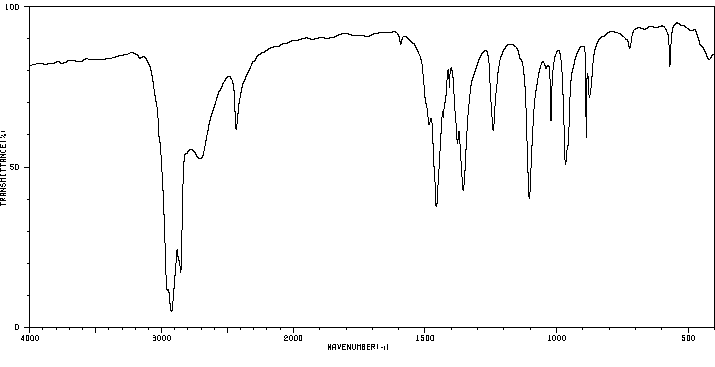
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷