2-Carboxy-naphthalsaeure-anhydrid | 36440-80-9
中文名称
——
中文别名
——
英文名称
2-Carboxy-naphthalsaeure-anhydrid
英文别名
2,4-Dioxo-3-oxatricyclo[7.3.1.05,13]trideca-1(12),5,7,9(13),10-pentaene-6-carboxylic acid;2,4-dioxo-3-oxatricyclo[7.3.1.05,13]trideca-1(12),5,7,9(13),10-pentaene-6-carboxylic acid
CAS
36440-80-9
化学式
C13H6O5
mdl
——
分子量
242.188
InChiKey
QOMBDAHFJRNHDB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:18
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:80.7
-
氢给体数:1
-
氢受体数:5
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 1,2-dihydroacenaphthylene-3-carboxylic acid 7424-63-7 C13H10O2 198.221
反应信息
-
作为反应物:描述:参考文献:名称:将稳定的酰胺转变成高反应活性的酰胺:捕获酶催化作用的本质摘要:天冬氨酸蛋白酶(包括HIV-1蛋白酶)在活性位点具有两个天冬氨酸羧基。已经在具有位于两个羧基之间的否则未活化的酰胺的系统中对这种关系进行了建模。模型酰胺以类似于酶的速率裂解,由于羧基和羧酸根基团的共同存在,使得酰胺在35°C和pH 4下不可分离。当存在合适的原子间相互作用时,将酶的几乎全部催化能力归因于静电重组的当前先进理论被证明是多余的。我们的动力学结果与时空概念一致,即将酰胺基团以适当的几何形状嵌入两个羧基部分之间,且距离小于水的直径,从而导致类酶速率增加。DOI:10.1002/anie.201701306
-
作为产物:描述:1,2-dihydroacenaphthylene-3-carboxylic acid 在 sodium dichromate 、 溶剂黄146 作用下, 生成 2-Carboxy-naphthalsaeure-anhydrid参考文献:名称:Synthesis of 1′,9-Methylene-1,2-benzanthracene and Related Hydrocarbons摘要:DOI:10.1021/ja01876a031
表征谱图
-
氢谱1HNMR
-
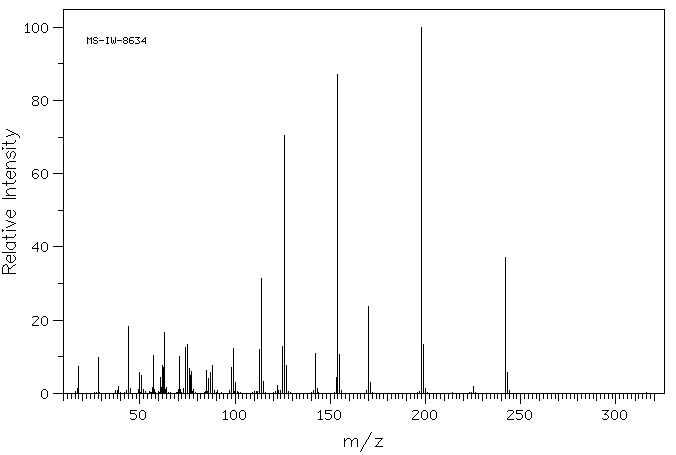
质谱MS
-
碳谱13CNMR
-
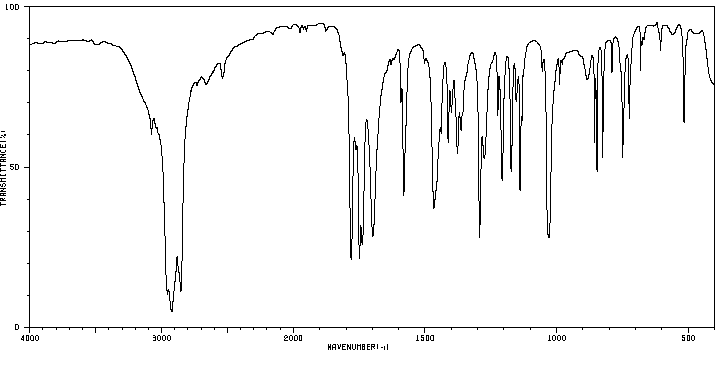
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮