Naphthalin-1,2,6,7-tetracarbonsaeure-tetramethylester | 68267-08-3
中文名称
——
中文别名
——
英文名称
Naphthalin-1,2,6,7-tetracarbonsaeure-tetramethylester
英文别名
tetramethyl naphthalene-1,2,6,7-tetracarboxylate;Tetramethyl 1,2,6,7-naphthalenetetracarboxylate
CAS
68267-08-3
化学式
C18H16O8
mdl
——
分子量
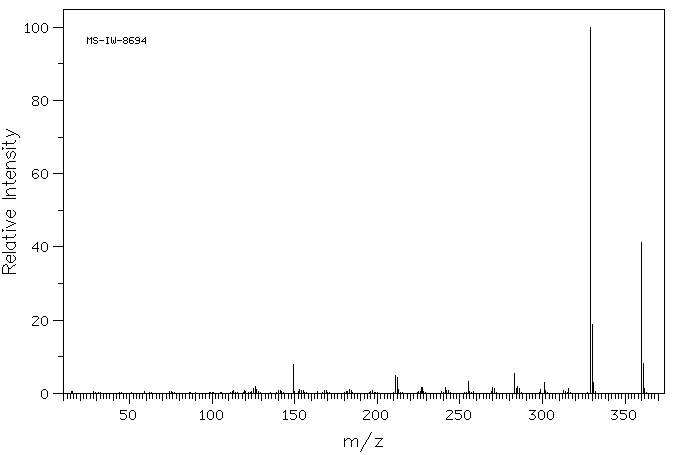
360.32
InChiKey
PURZTEAUNHESBR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:26
-
可旋转键数:8
-
环数:2.0
-
sp3杂化的碳原子比例:0.22
-
拓扑面积:105
-
氢给体数:0
-
氢受体数:8
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,2,6,7-tetrakis(hydroxymethyl)naphthalene 1296775-27-3 C14H16O4 248.279
反应信息
-
作为反应物:描述:Naphthalin-1,2,6,7-tetracarbonsaeure-tetramethylester 在 lithium aluminium tetrahydride 、 溴 、 三苯基膦 、 锌 作用下, 以 四氢呋喃 、 1,4-二氧六环 、 二氯甲烷 为溶剂, 反应 12.0h, 生成 1,2,3,4,8,9,10,11-octahydrobenz[a]anthracene-2,3,9,10-tetracarboxylic acid dianhydride参考文献:名称:来自 Dendralenes 的高度官能化、成角芳族化合物摘要:[3] 树枝烯 (2) 已转化为苯并 [a] 蒽四酯 13,该方案涉及二烯传输的 Diels-Alder 将乙炔二羧酸二甲酯加成到 2,然后进行一系列还原-加成-芳构化反应。同样,下一个更高的乙烯基 [4] 枝烯 (19) 提供了菲六酯 21。制备双邻二甲苯中间体 17 的尝试失败了。DOI:10.1002/ejoc.201001536
-
作为产物:描述:1,2,6,7-tetrakis(methoxycarbonyl)-3,5,8,8a-tetrahydronaphthalene 在 2,3-二氯-5,6-二氰基-1,4-苯醌 作用下, 以 甲苯 为溶剂, 反应 24.0h, 以54%的产率得到Naphthalin-1,2,6,7-tetracarbonsaeure-tetramethylester参考文献:名称:来自 Dendralenes 的高度官能化、成角芳族化合物摘要:[3] 树枝烯 (2) 已转化为苯并 [a] 蒽四酯 13,该方案涉及二烯传输的 Diels-Alder 将乙炔二羧酸二甲酯加成到 2,然后进行一系列还原-加成-芳构化反应。同样,下一个更高的乙烯基 [4] 枝烯 (19) 提供了菲六酯 21。制备双邻二甲苯中间体 17 的尝试失败了。DOI:10.1002/ejoc.201001536
文献信息
-
Synthesis and Consecutive Double Diels–Alder Cycloadditions of 3-Methylene-5-phenylsulfinyl-1-pentene as a Synthetic Equivalent of Parent Cross-conjugated Triene, 3-Methylene-1,4-pentadiene作者:Eiji Wada、Noritaka Nagasaki、Shuji Kanemasa、Otohiko TsugeDOI:10.1246/cl.1986.1491日期:1986.9.53-Methylene-5-phenylsulfinyl-1-pentene undergoes stepwise double Diels–Alder cycloadditions with two different dienophiles to afford hydronaphthalene skeletons. This sequence corresponds to cross type of diene-transmissive Diels-Alder cycloaddition of parent 3-methylene-1,4-pentadiene.
-
WADA EIJI; NAGASAKI NORITAKA; KANEMASA SHUJI; TSUGE OTOHIKO, CHEM. LETT.,(1986) N 9, 1491-1494作者:WADA EIJI、 NAGASAKI NORITAKA、 KANEMASA SHUJI、 TSUGE OTOHIKODOI:——日期:——
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
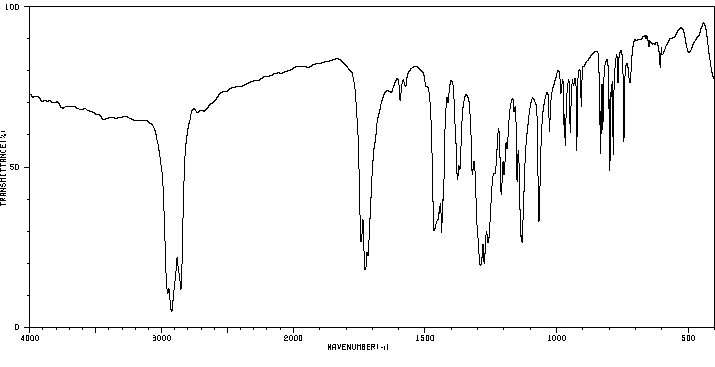
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮