8-氮杂二环[3.2.1]辛烷-3-酮,6-羟基-8-甲基- | 110061-26-2
中文名称
8-氮杂二环[3.2.1]辛烷-3-酮,6-羟基-8-甲基-
中文别名
——
英文名称
6β-hydroxytropinone
英文别名
6-hydroxytropinone;7-hydroxytropinone;6-Hydroxy-8-methyl-8-azabicyclo[3.2.1]octan-3-one
CAS
110061-26-2
化学式
C8H13NO2
mdl
MFCD00005550
分子量
155.197
InChiKey
UOHSTKWPZWFYTF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:123 °C
-
沸点:110-120 °C(Press: 0.5 Torr)
-
密度:1.217±0.06 g/cm3(Predicted)
-
保留指数:1325
计算性质
-
辛醇/水分配系数(LogP):-0.6
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.875
-
拓扑面积:40.5
-
氢给体数:1
-
氢受体数:3
SDS
上下游信息
反应信息
-
作为反应物:描述:8-氮杂二环[3.2.1]辛烷-3-酮,6-羟基-8-甲基- 在 磷酸 、 对甲苯磺酸 、 N,N'-二环己基碳二亚胺 作用下, 以 二甲基亚砜 、 苯 为溶剂, 反应 12.0h, 生成 8-Methyl-6H-spiro[8-azabicyclo[3.2.1]octane-3,2'-[1,3]dioxolan]-6-one参考文献:名称:Synthesis and Stereochemistry of Tropane 6-Spiro-hydantoins摘要:The synthesis of several representatives of the previously unknown N-alkyl-nortropane-6-spirohydantoin ring system by Bucherer-Bergs reaction of 8-alkyl-8-azabicyclo[3.2.1]octan-6-ones is described. 3-Oxo derivatives of the parent structure were also prepared. The stereochemical outcome of the Bucherer-Bergs reaction is discussed on the basis of H-1-nmr and C-13-nmr data.DOI:10.3987/com-92-6078
-
作为产物:参考文献:名称:DEWAR, G. H.;PARFITT, R. T.;SHEH, L., EUR. J. MED. CHEM., 1985, 20, N 3, 228-234摘要:DOI:
文献信息
-
[EN] PHENYL-HETEROARYL DERIVATIVES AND METHODS OF USE THEREOF<br/>[FR] DÉRIVÉS DE PHÉNYL-HÉTÉROARYLE ET PROCÉDÉS D'UTILISATION DE CEUX-CI申请人:TRANSTECH PHARMA INC公开号:WO2011103091A1公开(公告)日:2011-08-25The present invention provides phenyl-heteroaryl derivatives of Formula (I) and pharmaceutically acceptable salts thereof. These compounds are useful in the treatment of RAGE-mediated diseases such as Alzheimer's Disease. The present invention further relates to methods for the preparation of compounds of Formula (I) and pharmaceutically acceptable salts thereof, pharmaceutical compositions comprising such compounds, and the use of such compounds and/or pharmaceutical compositions in treating RAGE-mediated diseases.本发明提供了式(I)的苯基-杂环芳基衍生物及其药学上可接受的盐。这些化合物在治疗RAGE介导的疾病,如阿尔茨海默病方面是有用的。本发明还涉及制备式(I)化合物及其药学上可接受的盐的方法,包括含有这些化合物的药物组合物,以及在治疗RAGE介导的疾病中使用这些化合物和/或药物组合物的用途。
-
Specificities of the enzymes of N-alkyltropane biosynthesis in Brugmansia and Datura作者:Henry D. Boswell、Birgit Dräger、W.Russell McLauchlan、Andreas Portsteffen、David J. Robins、Richard J. Robins、Nicholas J. WaltonDOI:10.1016/s0031-9422(99)00293-9日期:1999.11The enzymes N-methylputrescine oxidase (MPO), the tropine-forming tropinone reductase (TRI), the pseudotropine-forming tropinone reductase (TRII), the tropine:acyl-CoA transferase (TAT) and the pseudotropine:acyl-CoA transferase (PAT) extracted from transformed root cultures of Datura stramonium and a Brugmansia candida x aurea hybrid were tested for their ability to accept a range of alternative substrates酶 N-甲基腐胺氧化酶 (MPO)、形成托品的托品酮还原酶 (TRI)、形成假托品的托品酮还原酶 (TRII)、托品:酰基辅酶 A 转移酶 (TAT) 和拟托品:酰基辅酶 A 转移酶 (PAT) ) 从曼陀罗和 Brugmansia candida x aurea 杂交体的转化根培养物中提取的 ) 接受了一系列替代底物的能力进行了测试。MPO 活性用 N-烷基腐胺和 N-烷基尸胺作为底物进行测试。针对一系列 N-烷基去甲托品酮、N-烷基去甲片碱和结构相关的酮作为底物测试了 TRI 和 TRII 还原。TAT 和 PAT 酯化测试使用一系列 N 取代的托品、拟托品、丸剂和拟丸剂作为底物来评估它们各自的乙酰基酯和 tigloyl 酯的形成。结果通常表明,这些酶会在不同程度上接受外来底物。这样的研究可能会揭示有关酶的活性位点的整体拓扑结构。
-
Norcaradiene intermediates in mass spectral fragmentations of tropone and tropothioneElectronic supplementary information (ESI) available: reaction paths supporting Figs. 3–6. See http://www.rsc.org/suppdata/p2/b1/b102127n/作者:Akihiro Ishiwata、Shinichi Yamabe、Tsutomu Minato、Takahisa MachiguchiDOI:10.1039/b102127n日期:2001.11.1The fragmentation of mass spectroscopy of tropone (1) was compared with that of tropothione (2). The electron-impact mass spectroscopy of 1 afforded almost exclusively the benzene cation radical (m/z = 78). Mass spectroscopy of 2 gave similarly the m/z = 78 base peak along with an unusual (M+ − 1) peak. This hydrogen elimination was found to occur at the C(α)–H bond by the use of a 2H-labeled analogue of 2. Ab initio calculations (UB3LYP/6-31G*) showed that a π cation radical of 1 and a σ cation radical of 2 were converted to norcaradiene intermediates. Their further isomerizations led to [the benzene cation radical (m/z = 78) + CO] and [(m/z = 78) + CS], respectively. The fragmentation channel of 1 was calculated to have sufficiently small activation energies of intervening transition states to give almost exclusively the m/z = 78 peak. For a σ radical of 2, an α hydrogen moved to the sulfur atom. The resultant thiol was isomerized to a second norcaradiene and its further isomerization led to a thioketene like cation and a hydrogen atom corresponding to the unusual (M+ − 1) peak. The difference in fragmentation patterns of 1 and 2 is discussed in terms of their electronic structures.将托品酮(1)的质谱碎片与托品硫酮(2)的质谱碎片进行了比较。1 的电子撞击质谱几乎只提供苯阳离子基(m/z = 78)。2 的质谱同样显示出 m/z = 78 的基峰和一个不寻常的 (M+ - 1) 峰。Ab initio 计算(UB3LYP/6-31G*)表明,1 的 π 阳离子自由基和 2 的 σ 阳离子自由基转化成了去甲二苯中间体。它们的进一步异构化分别导致了[苯阳离子基 (m/z = 78) + CO] 和[(m/z = 78) + CS]。根据计算,1 的碎片通道具有足够小的中间过渡态活化能,几乎只产生 m/z = 78 峰。对于 2 的 σ 自由基,α 氢转移到了硫原子上。由此产生的硫醇被异构化为第二个壬二醛,其进一步异构化产生了一个类似硫酮的阳离子和一个氢原子,对应于不寻常的 (M+ - 1) 峰。我们从电子结构的角度讨论了 1 和 2 碎片模式的差异。
-
Bicyclic amine derivatives申请人:——公开号:US20020061913A1公开(公告)日:2002-05-23The present invention provides compounds of formula (I): 1 wherein R, A, Ar and R 1 are as defined in the specification. The invention also provides insecticidal, acaricidal and nematicidal compositions comprising the compound of formula (I), and methods for combating and controlling insect, acarine and nematdoe pests at a locus by treating the pests or the locus with an effective amount of the compound of formula (I) or compositions thereof.本发明提供了式(I)的化合物:1其中R、A、Ar和R1如规范中所定义。该发明还提供了包含式(I)化合物的杀虫、杀螨和杀线虫组合物,以及通过使用式(I)化合物或其组合物的有效量来处理害虫或处理地点以对抗和控制昆虫、螨和线虫害虫的方法。
-
PHENYL-HETEROARYL DERIVATIVES AND METHODS OF USE THEREOF申请人:Gohimukkula Devi R.公开号:US20110230458A1公开(公告)日:2011-09-22The present invention provides phenyl-heteroaryl derivatives of Formula (I) and pharmaceutically acceptable salts thereof These compounds are useful in the treatment of RAGE-mediated diseases such as Alzheimer's Disease. The present invention further relates to methods for the preparation of compounds of Formula (I) and pharmaceutically acceptable salts thereof, pharmaceutical compositions comprising such compounds, and the use of such compounds and/or pharmaceutical compositions in treating RAGE-mediated diseases.本发明提供了式(I)的苯基-杂环取代衍生物及其药学上可接受的盐。这些化合物在治疗RAGE介导的疾病,如阿尔茨海默病中是有用的。本发明还涉及制备式(I)化合物及其药学上可接受的盐的方法,包括这些化合物的制药组合物,以及在治疗RAGE介导的疾病时使用这些化合物和/或制药组合物。
表征谱图
-
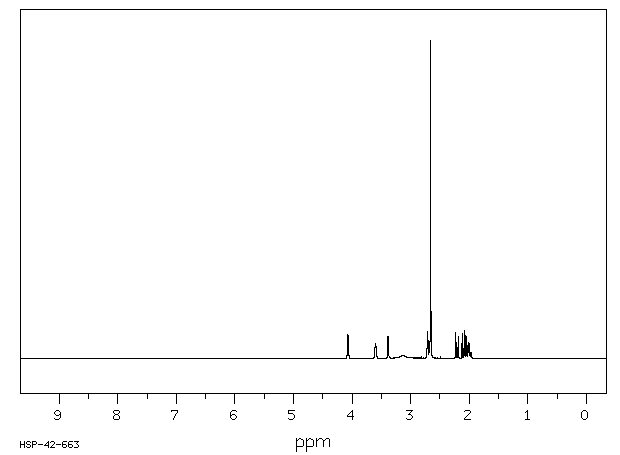
氢谱1HNMR
-
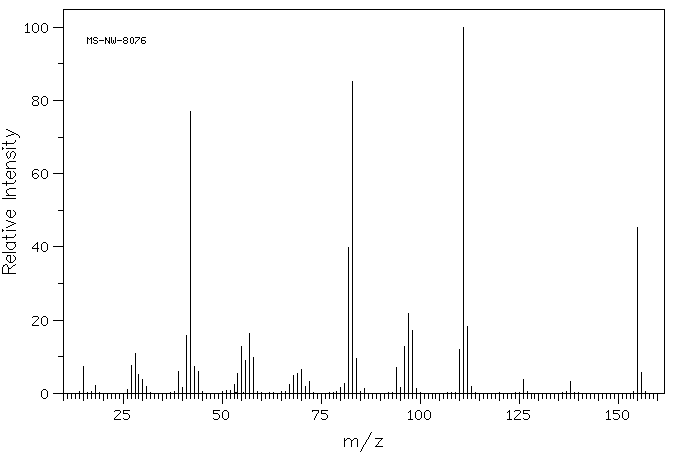
质谱MS
-
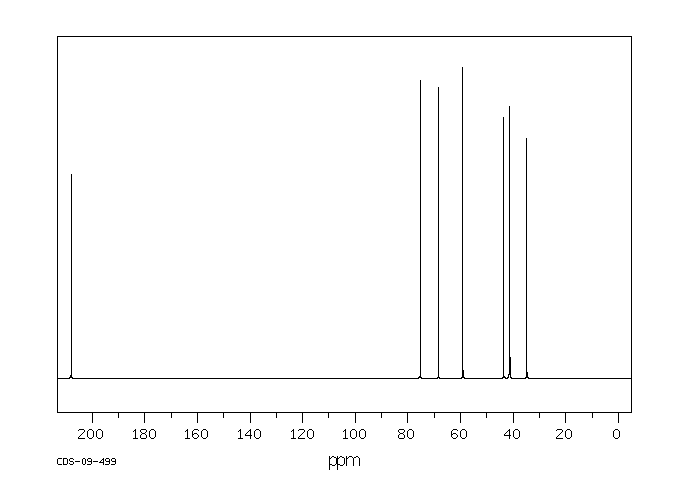
碳谱13CNMR
-
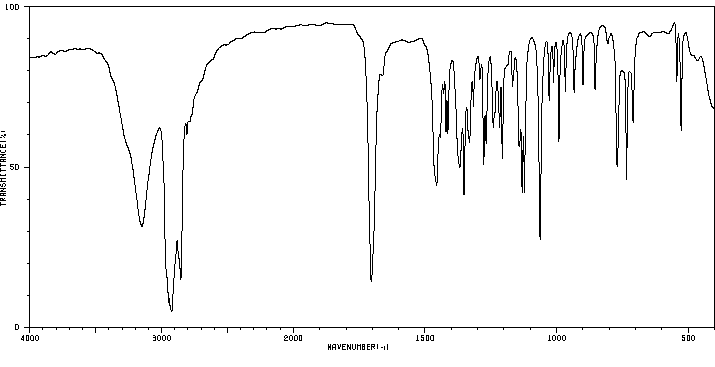
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
马拉维若
降莨菪鹼
阿托美品
阿托品硫酸盐
阿托品杂质6
阿托品EP杂质E
辛托溴铵
西克托溴铵
莨菪鹼鹽酸鹽
莨菪碱
莨菪烷
苯酰胺,2-乙氧基-N-[[(8-甲基-8-氮杂二环[3.2.1]辛-3-基)氨基]羰基]-,内-
苯酰胺,2-丁氧基-N-[[(8-甲基-8-氮杂二环[3.2.1]辛-3-基)氨基]羰基]-,内-
苯甲胺,3,5-二氯-N-甲基-
苯甲基2-乙酰氨基-3,6-DI-O-苯甲酰-2-脱氧-α-D-吡喃半乳糖苷
苯基(1R)-3-(4-碘苯基)-8-甲基-8-氮杂双环[3.2.1]辛烷-2-羧酸酯
芽子碱盐酸盐
芽子碱甲酯
芽子碱甲基酯盐酸盐
芽子碱
碘氟潘
硫酸莨菪碱水合物
硫酸茛菪素
盐酸去甲托品
白曼陀罗碱
甲硫托铵
甲基8-甲基-3-苯基-8-氮杂双环[3.2.1]辛烷-2-羧酸酯
甲基(1R,2S,3S,5S)-3-(4-氯苯基)-8-[(Z)-3-碘丙-2-烯基]-8-氮杂双环[3.2.1]辛烷-2-羧酸酯
甲基(1R,2S,3S,5S)-3-(4-氟苯基)-8-甲基-8-氮杂双环[3.2.1]辛烷-2-甲酸酯
甲基(1R,2S,3S,5S)-3-(4-氟苯基)-8-丙-2-烯基-8-氮杂双环[3.2.1]辛烷-2-羧酸酯
甲基(1R)-3-(4-氯苯基)-8-甲基-8-氮杂双环[3.2.1]辛烷-2-羧酸酯
甲基(1R)-3-(4-氟苯基)-8-丙基-8-氮杂双环[3.2.1]辛烷-2-羧酸酯
瑞他莫林杂质7
瑞他莫林中间体
环戊烯,1,5-二甲基-3,4-二(亚甲基)-2-(1-甲基乙基)-(9CI)
环己醇并,2-[(4-乙基苯基)氨基]-,(1R,2R)-rel-
特索芬辛
泽泊思定
氢溴酸天仙子胺
氢溴酸天仙子胺
暂无
斯泰因N1
托品醇异丁酸酯
托品醇壬酸酯
托品醇-3-黄酸酯
托品醇
托品酮
托品巴酯
托品-3-硫醇
打碗花精A5