(24ξ)-241-methoxy-24-methylcycloartane-3β,24-diol
分子结构分类
中文名称
——
中文别名
——
英文名称
(24ξ)-241-methoxy-24-methylcycloartane-3β,24-diol
英文别名
(24R,S)-241-methoxy-24-methylcycloartane-3β,24-diol;(1S,3R,6S,8R,11S,12S,15R,16R)-15-[(2R)-5-hydroxy-5-(methoxymethyl)-6-methylheptan-2-yl]-7,7,12,16-tetramethylpentacyclo[9.7.0.01,3.03,8.012,16]octadecan-6-ol
CAS
——
化学式
C32H56O3
mdl
——
分子量
488.795
InChiKey
BGKDKAMMXBHXAE-YGBPDJJZSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):8.4
-
重原子数:35
-
可旋转键数:7
-
环数:5.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:49.7
-
氢给体数:2
-
氢受体数:3
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 24-亚甲基环阿屯醇 24-methylenecycloartanol 1449-09-8 C31H52O 440.753
反应信息
-
作为产物:描述:24-亚甲基环阿屯醇 在 Glomerella fusarioides IFO 8831 mycelium 作用下, 以 甲醇 、 二甲基亚砜 为溶剂, 反应 240.0h, 以2.6%的产率得到(24ξ)-241-methoxy-24-methylcycloartane-3β,24-diol参考文献:名称:Biotransformation of Cycloartane-Type Triterpenes by the Fungus Glomerella fusarioides摘要:Biotransformation of three cycloartane-type triterpenes, cycloartenol (1), 24-methylenecycloartanol (2), and cycloartenone (3), by the fungus Glomerella fusarioides was studied. Compound 1 was converted to 3, cycloart-25-ene-3 beta, 24-diol (4), and cycloartane-3 beta, 24,25-triol (5). Compound 2 was metabolized to cycloeucalenol (6) and two new compounds, 24-methylcycloartane-3 beta,24,24(1)-triol (7) and 24(1)-methoxy-24-methylcycloartane-3 beta, 24-diol (8). Compound 3 was converted into two new metabolites, 4 alpha,4 beta,14 alpha-trimethyl-9 beta, 19-cyclopregnane-3,20-dione (9) and 25-hydroxy-24-methoxycycloartan-3-one (14), and four known compounds, viz., cycloartane-3,24-dione (10), 24-hydroxycycloart-25-en-3-one (11), (23E)-25-hydroxycycloart-23-en-3-one (12), and 24,25-dihydroxycycloartan-3-one (13). The structures of four new metabolites, 7, 8, 9, and 14, were established by spectroscopic methods.DOI:10.1021/np058120d
表征谱图
-
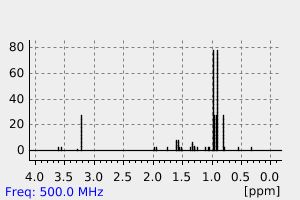
氢谱1HNMR
-
质谱MS
-
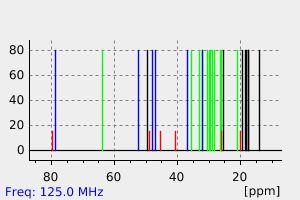
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β)-17,20:20,21-双[亚甲基双(氧基)]孕烷-3-酮
(5α)-2′H-雄甾-2-烯并[3,2-c]吡唑-17-酮
(3β,20S)-4,4,20-三甲基-21-[[[三(异丙基)甲硅烷基]氧基]-孕烷-5-烯-3-醇-d6
(25S)-δ7-大发酸
(20R)-孕烯-4-烯-3,17,20-三醇
(11β,17β)-11-[4-({5-[(4,4,5,5,5-五氟戊基)磺酰基]戊基}氧基)苯基]雌二醇-1,3,5(10)-三烯-3,17-二醇
齐墩果酸衍生物1
黄麻属甙
黄芪皂苷III
黄芪皂苷 II
黄芪甲苷 IV
黄芪甲苷
黄肉楠碱
黄果茄甾醇
黄杨醇碱E
黄姜A
黄夹苷B
黄夹苷
黄夹次甙乙
黄夹次甙乙
黄夹次甙丙
黄体酮环20-(乙烯缩醛)
黄体酮杂质EPL
黄体酮杂质1
黄体酮杂质
黄体酮杂质
黄体酮EP杂质M
黄体酮EP杂质G(RRT≈2.53)
黄体酮EP杂质F
黄体酮6-半琥珀酸酯
黄体酮 17alpha-氢过氧化物
黄体酮 11-半琥珀酸酯
黄体酮
麦角甾醇葡萄糖苷
麦角甾醇氢琥珀酸盐
麦角甾烷-6-酮,2,3-环氧-22,23-二羟基-,(2b,3b,5a,22R,23R,24S)-(9CI)
麦角甾烷-3,6,8,15,16-五唑,28-[[2-O-(2,4-二-O-甲基-b-D-吡喃木糖基)-a-L-呋喃阿拉伯糖基]氧代]-,(3b,5a,6a,15b,16b,24x)-(9CI)
麦角甾烷-26-酸,5,6:24,25-二环氧-14,17,22-三羟基-1-羰基-,d-内酯,(5b,6b,14b,17a,22R,24S,25S)-(9CI)
麦角甾-8-烯-3-醇
麦角甾-8,24(28)-二烯-26-酸,7-羟基-4-甲基-3,11-二羰基-,(4a,5a,7b,25S)-
麦角甾-7,22-二烯-3-酮
麦角甾-7,22-二烯-17-醇-3-酮
麦角甾-5,24-二烯-26-酸,3-(b-D-吡喃葡萄糖氧基)-1,22,27-三羟基-,d-内酯,(1a,3b,22R)-
麦角甾-5,22,25-三烯-3-醇
麦角甾-4,6,8(14),22-四烯-3-酮
麦角甾-1,4-二烯-3-酮,7,24-二(乙酰氧基)-17,22-环氧-16,25-二羟基-,(7a,16b,22R)-(9CI)
麦角固醇
麦冬皂苷D
麦冬皂苷D
麦冬皂苷 B