顺式-1,2-二苯基环丁烷 | 7694-30-6
中文名称
顺式-1,2-二苯基环丁烷
中文别名
——
英文名称
cis-1,2-diphenylcyclobutane
英文别名
cis-1,2-Diphenylcyclobutan;1,2-diphenylcyclobutane;syn-1,2-diphenylcyclobutane;[(1S,2R)-2-phenylcyclobutyl]benzene
CAS
7694-30-6
化学式
C16H16
mdl
——
分子量
208.303
InChiKey
AERGGMDNGDDGPI-IYBDPMFKSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:309.1±22.0 °C(Predicted)
-
密度:1.044±0.06 g/cm3(Predicted)
-
溶解度:可溶于氯仿;二氯甲烷;甲醇
-
保留指数:1640
计算性质
-
辛醇/水分配系数(LogP):4.6
-
重原子数:16
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— trans-1,2-Diphenyl-cyclobutan —— C16H16 208.303 反式-1,2-二苯基环丁烷-D5 trans-1,2-Diphenyl-cyclobutan 3018-21-1 C16H16 208.303
反应信息
-
作为反应物:描述:参考文献:名称:Proton catalyzed cis-trans stereomutation of cis-1,2-diarylcyclobutanes摘要:DOI:10.1021/ja00275a073
-
作为产物:描述:参考文献:名称:Kaupp,G.; Stark,M., Chemische Berichte, 1977, vol. 110, p. 3084 - 3110摘要:DOI:
文献信息
-
Photosensitised regioselective [2+2]-cycloaddition of cinnamates and related alkenes作者:Santosh K. Pagire、Asik Hossain、Lukas Traub、Sabine Kerres、Oliver ReiserDOI:10.1039/c7cc06710k日期:——An efficient method for the synthesis of substituted cyclobutanes from cinnamates, chalcones, and styrenes has been developed utilizing a visible-light triplet sensitisation mode. This reaction provides a diverse range of substituted cyclobutanes in high yields under mild conditions without the need of external additives. Good regioselectivity is obtained due to strong π–π-stacking of arene moieties
-
Visible light-induced highly selective transformation of olefin to ketone by 2,4,6-triphenylpyrylium cation encapsulated within zeolite Y作者:Yasuhiro Shiraishi、Naoya Saito、Takayuki HiraiDOI:10.1039/b515137f日期:——2,4,6-Triphenylpyrylium cation encapsulated within zeolite Y promotes highly selective transformation of olefins to ketones with molecular oxygen, under visible light (lambda > 400 nm) irradiation at room temperature.在室温下在可见光(λ> 400 nm)照射下,包裹在沸石Y中的2,4,6-三苯基吡啶鎓阳离子可促进烯烃与分子氧的高选择性转化为酮。
-
Reductive Carbocyclization of Homoallylic Alcohols to <i>syn</i> -Cyclobutanes by a Boron-Catalyzed Dual Ring-Closing Pathway作者:Chinmoy Kumar Hazra、Jinhoon Jeong、Hyunjoong Kim、Mu-Hyun Baik、Sehoon Park、Sukbok ChangDOI:10.1002/anie.201713285日期:2018.3.1carbocyclization of homoallylic alcohols has been developed by using hydrosilanes as reducing reagents to provide a range of 1,2‐disubstituted arylcyclobutanes. The reaction proceeds in a cis‐selective manner with high efficiency under mild conditions. Mechanistic studies, including deuterium scrambling and Hammett studies, and DFT calculations, suggest a dual ring‐closing pathway.
-
The synthesis of cycloalkanes via anionic coupling reactions作者:D. H. Richards、N. F. ScillyDOI:10.1039/j39690002661日期:——A number of cycloalkanes have been isolated among the products of the reactions of geminal and vicinal dihalides with dimeric dianions produced from substituted styrenes by the action of lithium. The n.m.r. and mass spectra of the products are reported.
-
SINGLET-PHOTOSENSITIZED RING-SPLITTING AND ISOMERIZATION REACTIONS OF 1,2-DIPHENYLCYCLOBUTANE BY AROMATIC NITRILES. A POSSIBLE PROBE FOR RELATIONSHIPS BETWEEN REACTIVITIES AND ELECTRONIC NATURE OF EXCIPLEX INTERMEDIATES作者:Chyongjin Pac、Takao Fukunaga、Tomohito Ohtsuki、Hiroshi SakuraiDOI:10.1246/cl.1984.1847日期:1984.10.5The photosensitized reactions of cis-1,2-diphenylcyclobutane by 1,4-dicyanonaphthalene and 9,10-dicyanoanthracene gave styrene, the trans isomer, and 1-phenyltetralin whereas the photosensitization by 1-cyanonaphthalene effected the ring-splitting and cis,trans-isomerization reactions without the formation of 1-phenyltetralin. Similar reactions of the trans isomer were photosensitized by the aromatic
表征谱图
-
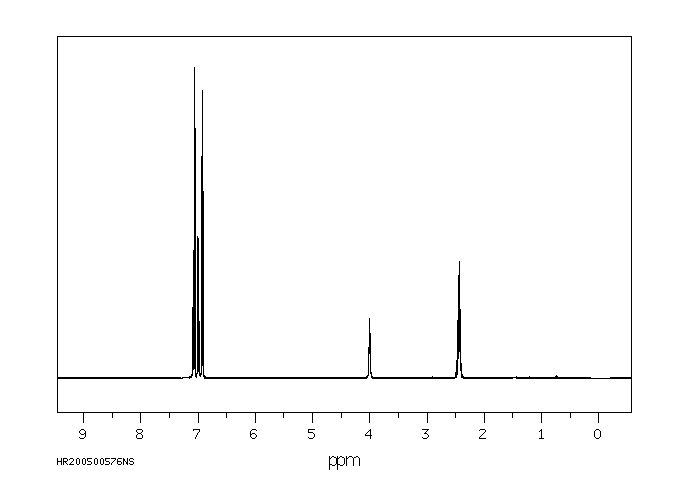
氢谱1HNMR
-
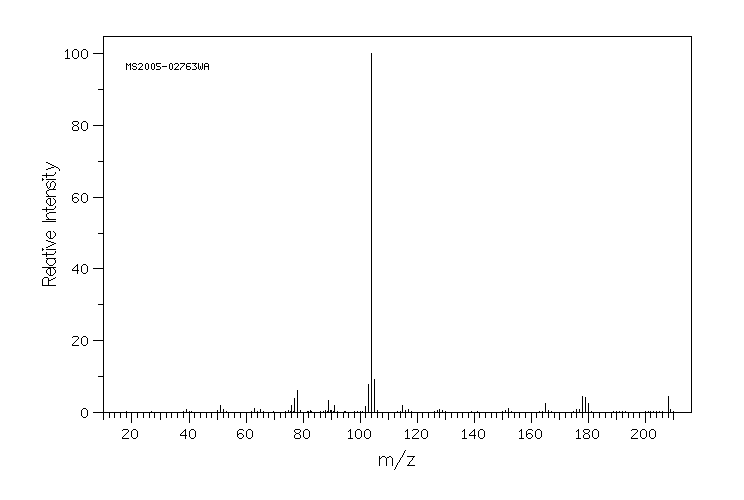
质谱MS
-
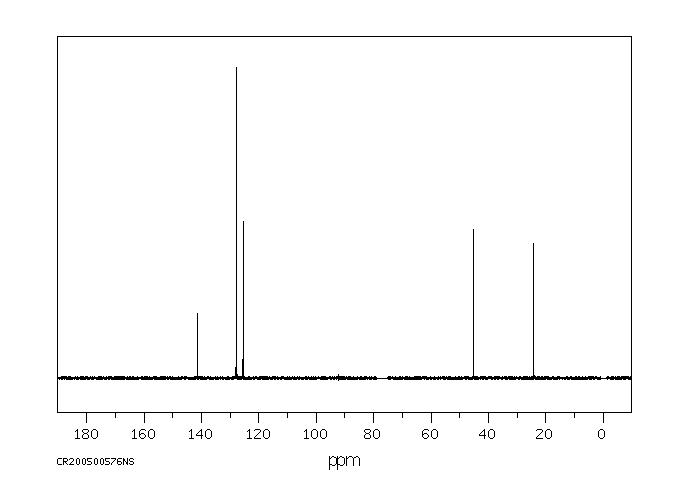
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E,Z)-他莫昔芬N-β-D-葡糖醛酸
(E/Z)-他莫昔芬-d5
(4S,5R)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S,5R,5''R)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(4R,5S)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4R,4''R,5S,5''S)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(1R,2R)-2-(二苯基膦基)-1,2-二苯基乙胺
鼓槌石斛素
黄子囊素
高黄绿酸
顺式白藜芦醇三甲醚
顺式白藜芦醇
顺式己烯雌酚
顺式-白藜芦醇3-O-beta-D-葡糖苷酸
顺式-桑皮苷A
顺式-曲札芪苷
顺式-二苯乙烯
顺式-beta-羟基他莫昔芬
顺式-a-羟基他莫昔芬
顺式-3,4',5-三甲氧基-3'-羟基二苯乙烯
顺式-1-(3-甲基-2-萘基)-2-(2-萘基)乙烯
顺式-1,2-双(三甲基硅氧基)-1,2-双(4-溴苯基)环丙烷
顺式-1,2-二苯基环丁烷
顺-均二苯乙烯硼酸二乙醇胺酯
顺-4-硝基二苯乙烯
顺-1-异丙基-2,3-二苯基氮丙啶
非洲李(PRUNUSAFRICANA)树皮提取物
阿非昔芬
阿里可拉唑
阿那曲唑二聚体
阿托伐他汀环氧四氢呋喃
阿托伐他汀环氧乙烷杂质
阿托伐他汀环(氟苯基)钠盐杂质
阿托伐他汀环(氟苯基)烯丙基酯
阿托伐他汀杂质D
阿托伐他汀杂质94
阿托伐他汀杂质7
阿托伐他汀杂质5
阿托伐他汀内酰胺钠盐杂质
阿托伐他汀中间体M4
阿奈库碘铵
锌(II)(苯甲醛)(四苯基卟啉)
银松素
铜酸盐(5-),[m-[2-[2-[1-[4-[2-[4-[[4-[[4-[2-[4-[4-[2-[2-(羧基-kO)苯基]二氮烯基-kN1]-4,5-二氢-3-甲基-5-(羰基-kO)-1H-吡唑-1-基]-2-硫代苯基]乙烯基]-3-硫代苯基]氨基]-6-(苯基氨基)-1,3,5-三嗪-2-基]氨基]-2-硫代苯基]乙烯基]-3-硫代
铒(III) 离子载体 I
铀,二(二苯基甲酮)四碘-
钾钠2,2'-[(E)-1,2-乙烯二基]二[5-({4-苯胺基-6-[(2-羟基乙基)氨基]-1,3,5-三嗪-2-基}氨基)苯磺酸酯](1:1:1)
钠{4-[氧代(苯基)乙酰基]苯基}甲烷磺酸酯
钠;[2-甲氧基-5-[2-(3,4,5-三甲氧基苯基)乙基]苯基]硫酸盐
钠4-氨基二苯乙烯-2-磺酸酯