1H,3H-萘并[1,8-cd]噻喃 | 203-85-0
中文名称
1H,3H-萘并[1,8-cd]噻喃
中文别名
1H,3H-萘并[1,8-CD]噻喃
英文名称
1H,3H-naphtho<1,8-cd>thiopyran
英文别名
1H,3H-Naphtho[1,8-cd]thiopyran;3-thiatricyclo[7.3.1.05,13]trideca-1(12),5,7,9(13),10-pentaene
CAS
203-85-0
化学式
C12H10S
mdl
——
分子量
186.277
InChiKey
PIUJLKSYKOKWMI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:372.4±31.0 °C(Predicted)
-
密度:1.224
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:13
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:25.3
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2930909090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1H,3H-Naphtho<1,8-cd>thiopyran 2,2-Dioxide 29376-61-2 C12H10O2S 218.276
反应信息
-
作为反应物:描述:1H,3H-萘并[1,8-cd]噻喃 在 N-溴代丁二酰亚胺(NBS) 、 溴 、 sodium methylate 、 1,8-二氮杂双环[5.4.0]十一碳-7-烯 、 间氯过氧苯甲酸 、 过氧化苯甲酰 作用下, 以 甲醇 、 四氯化碳 、 二氯甲烷 为溶剂, 反应 71.5h, 生成 1-bromoacenaphthylene参考文献:名称:Ramberg-Backlund reaction of 1,3-dibromo-1H,3H-naphtho[1,8-cd]thiopyran 2,2-dioxide. Formation of acenaphthyne intermediate摘要:DOI:10.1021/jo00149a013
-
作为产物:描述:参考文献:名称:Molecules with a helix structure. 3. Sulfur-bridged peri-naphthalenes: synthesis, conformational analysis, and photoelectron spectroscopy of the mono-, di-, and trisulfides of 1,8-dimethylnaphthalene摘要:DOI:10.1021/ja00391a029
文献信息
-
Hypervalent iodine(III)-mediated C(sp3) H bond arylation, alkylation, and amidation of isothiochroman作者:Wataru Muramatsu、Kimihiro NakanoDOI:10.1016/j.tetlet.2014.11.134日期:2015.1We have developed a one-step method for introducing aryl, alkyl, and amide groups at the C(1)-position of isothiochromans via an oxidation reaction using hypervalent iodine(III), [bis(trifluoroacetoxy)iodo]benzene (PIFA), followed by an nucleophilic addition reaction using Grignard reagents and an amide.
-
A facile, high-yielding method for the conversion of halides to mercaptans作者:Christopher Bieniarz、Michael J. CornwellDOI:10.1016/s0040-4039(00)77459-4日期:1993.2An efficient, high-yield, one-pot preparation of alkyl and activated aryl mercaptans is presented. The method relies on sodium thiophosphate displacement of the halide, followed by mild hydrolysis of the intermediate phosphorothioate.
-
The first stable tetraarylacenaphthenequinodimethanes exhibiting electrochromism with ‘write-protect’ option: preparation, highly deformed structure, and reversible interconversion with acenaphthylene-5,6-diyl dicationic dyes作者:Takanori Suzuki、Shinichi Iwashita、Tsuyoshi Yoshino、Hidetoshi Kawai、Kenshu Fujiwara、Masakazu Ohkita、Takashi Tsuji、Kazunori Ono、Masami TakenakaDOI:10.1016/j.tetlet.2005.11.059日期:2006.1Severely deformed title quinodimethanes [1,2-bis(diarylmethylene)acenaphthenes] 1 have been designed and prepared as novel electrochromic materials, which can be reversibly interconverted with the deeply colored dicationic dyes 1(2+) with acenaphthylene-1,2-diyl skeleton. The X-ray analysis of 1 revealed that the inner two aryl groups are forced to overlap in proximity, which can account for the facile electrocyclization process to the isomer. By combination of reversible electrochemical transformation with irreversible isomerization process, the present system provides a prototype for novel molecular response systems equipped with the 'write-protect' option. (c) 2005 Elsevier Ltd. All rights reserved.
-
NAKAYAMA, JUZO;OHSHIMA, ETSUO;ISHII, AKIHIKO;HOSHINO, MASAMATSU, J. ORG. CHEM., 1983, 48, N 1, 60-65作者:NAKAYAMA, JUZO、OHSHIMA, ETSUO、ISHII, AKIHIKO、HOSHINO, MASAMATSUDOI:——日期:——
-
ISOCHROMAN COMPOUNDS FOR TREATMENT OF CNS DISORDERS申请人:ELI LILLY AND COMPANY公开号:EP1458703B1公开(公告)日:2007-02-28
表征谱图
-
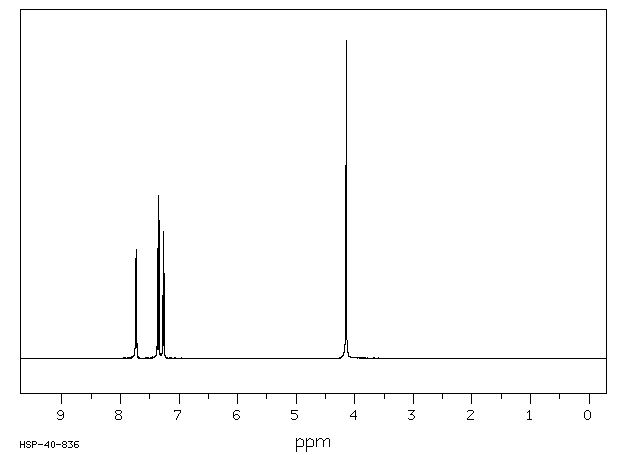
氢谱1HNMR
-
质谱MS
-
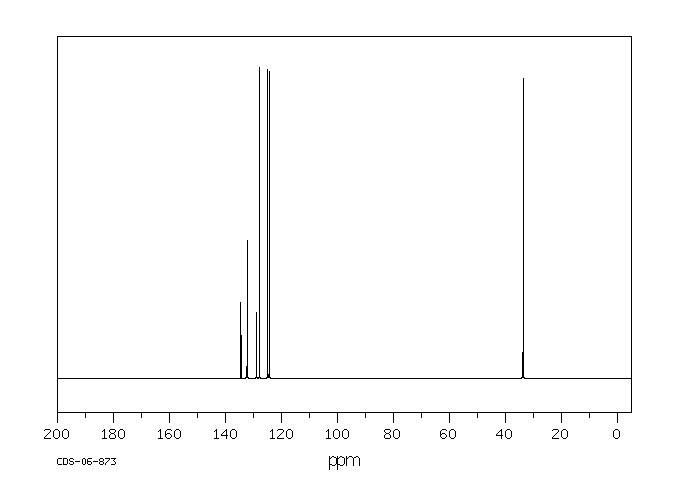
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮