3-溴-2,5-二氯噻吩 | 60404-18-4
中文名称
3-溴-2,5-二氯噻吩
中文别名
3-溴-2,5-二氯噻酚
英文名称
3-bromo-2,5-dichloro thiophene
英文别名
2,5-dichloro-3-bromothiophene;3-bromo-2,5-dichlorothiophene;3-bromo-2,5-dichloro-thiophene;3-Brom-2,5-dichlor-thiophen
CAS
60404-18-4
化学式
C4HBrCl2S
mdl
——
分子量
231.928
InChiKey
PBUHOXBSIQJRNO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:95-97°C 14mm
-
密度:1.949
-
闪点:95-97°C/14mm
-
稳定性/保质期:
避免接触氧化物
计算性质
-
辛醇/水分配系数(LogP):4.2
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:28.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
安全说明:S28,S36/37,S45
-
危险类别码:R23/24/25
-
海关编码:2934999090
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 3-Bromo-2,5-dichlorothiophene
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H301: Toxic if swallowed
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
Avoid breathing dust/fume/gas/mist/vapours/spray
P261:
P280: Wear protective gloves/protective clothing/eye protection/face protection
P301+P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P405: Store locked up
Section 3. Composition/information on ingredients.
Ingredient name: 3-Bromo-2,5-dichlorothiophene
CAS number: 60404-18-4
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C4HBrCl2S
Molecular weight: 231.9
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen chloride, hydrogen bromide, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 3-Bromo-2,5-dichlorothiophene
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H301: Toxic if swallowed
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
Avoid breathing dust/fume/gas/mist/vapours/spray
P261:
P280: Wear protective gloves/protective clothing/eye protection/face protection
P301+P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P405: Store locked up
Section 3. Composition/information on ingredients.
Ingredient name: 3-Bromo-2,5-dichlorothiophene
CAS number: 60404-18-4
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C4HBrCl2S
Molecular weight: 231.9
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen chloride, hydrogen bromide, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氯-3-溴噻吩 3-bromo-2-chlorothiophene 40032-73-3 C4H2BrClS 197.483
反应信息
-
作为反应物:描述:参考文献:名称:一种氧化噻吩的新方法。含吸电子取代基的噻吩 1,1-二氧化物的合成摘要:开发了一种通过用三氟过氧乙酸氧化取代噻吩来合成噻吩 1,1-二氧化物的新方法。研究了溶剂性质对反应过程的影响,并找到了氧化含有各种官能团的噻吩的最佳条件。获得了以前未知的噻吩二氧化物。DOI:10.1007/s11172-005-0107-9
-
作为产物:参考文献:名称:[EN] 2-AMINO-N-(AMINO-OXO-ARYL-LAMBDA6-SULFANYLIDENE)ACETAMIDE COMPOUNDS AND THEIR THERAPEUTIC USE
[FR] COMPOSÉS DE 2-AMINO-N-(AMINO-OXO-ARYL-LAMBDA6-SULFANYLIDÈNE)ACÉTAMIDE ET LEUR UTILISATION THÉRAPEUTIQUE摘要:本发明一般涉及治疗化合物领域。更具体地,本发明涉及某些2-氨基-N-(氨基氧代芳基-λ6-砜基)乙酰胺化合物(以下简称为ANASIA化合物),该化合物在一些情况下抑制(例如,选择性抑制)细菌氨酰-tRNA合成酶(aaRS)(例如,细菌亮氨酰-tRNA合成酶,LeuRS)。本发明还涉及包含这种化合物的药物组合物,以及在体内外使用这种化合物和组合物来抑制(例如,选择性抑制)细菌氨酰-tRNA合成酶;治疗通过抑制(例如,选择性抑制)细菌氨酰-tRNA合成酶而得到改善的疾病;治疗细菌感染等。公开号:WO2021123237A1
文献信息
-
Novel aminophenyl ketone derivatives申请人:——公开号:US20030073832A1公开(公告)日:2003-04-17Novel heteroaryl aminophenyl ketone derivatives which are inhibitors of MAP kinases, in particular the p38 MAP kinase, are useful as anti-inflammatory agents in the prophylaxis or treatment of inflammatory diseases or conditions.
-
Hydronopol derivatives as agonists on human ORL1 receptors申请人:Mentzel Matthias公开号:US20050131004A1公开(公告)日:2005-06-16The invention relates to a group of hydronopol derivatives which are agonists on human ORL1 (nociceptin) receptors. The invention also relates to the preparation of these compounds, to pharmaceutical compositions containing a pharmacologically active amount of at least one of these novel hydronopol derivatives as an active ingredient, as well as to the use of these pharmaceutical compositions for the treatment of disorders in which ORL1 receptors are involved. The invention relates to compounds of the general formula (1) wherein the symbols have the meanings as given in the description.
-
HETERO-FUSED CYCLIC COMPOUND申请人:EISAI R&D MANAGEMENT CO., LTD.公开号:US20160168176A1公开(公告)日:2016-06-16A compound represented by the formula (I) or a salt thereof: wherein a ring Z is a 5 to 6-membered heteroaromatic ring having one or two heteroatoms in the ring; X 1 is a hydrogen atom, a hydroxy group, a hydroxy C 1-6 alkyl group, —B(OH) 2 , a boronate ester group, a cyclic boronate ester group, a boranyl group, a cyclic boranyl group, —BF 3 M n1 , —Sn(R 12 )(R 13 )(R 14 ), a leaving group, a carboxy group, a formyl group, or —NR 16 R 17 ; and X 2 is a hydrogen atom or —CO 2 R 18 .
-
Iridium-catalyzed borylation of thiophenes: versatile, synthetic elaboration founded on selective C–H functionalization作者:Ghayoor A. Chotana、Venkata A. Kallepalli、Robert E. Maleczka、Milton R. SmithDOI:10.1016/j.tet.2008.02.111日期:2008.6various substituted thiophenes to synthesize poly-functionalized thiophenes in good to excellent yields. Apart from common functionalities compatible with iridium-catalyzed borylations, additional functional group tolerance to acyl (COMe), and trimethylsilyl (TMS) groups was also observed. High regioselectivities were observed in borylation of 3-and 2,5-di-substituted thiophenes. Electrophilic aromatic
-
A facile route to thiophene-1,1-dioxides bearing electron-withdrawing groups作者:Valentine G Nenajdenko、Andrew E Gavryushin、Elizabeth S BalenkovaDOI:10.1016/s0040-4039(01)00732-8日期:2001.6thiophene-1,1-dioxides with strong EWGs has been described to date. Trifluoroperacetic acid in acetonitrile in the absence of water is shown to oxidise thiophenes, including examples possessing an electron-withdrawing group such as sulfonyl or alkoxycarbonyl. An easy and ready for scale-up procedure is developed, some formerly unknown thiophene-1,1-dioxides are obtained.
表征谱图
-
氢谱1HNMR
-
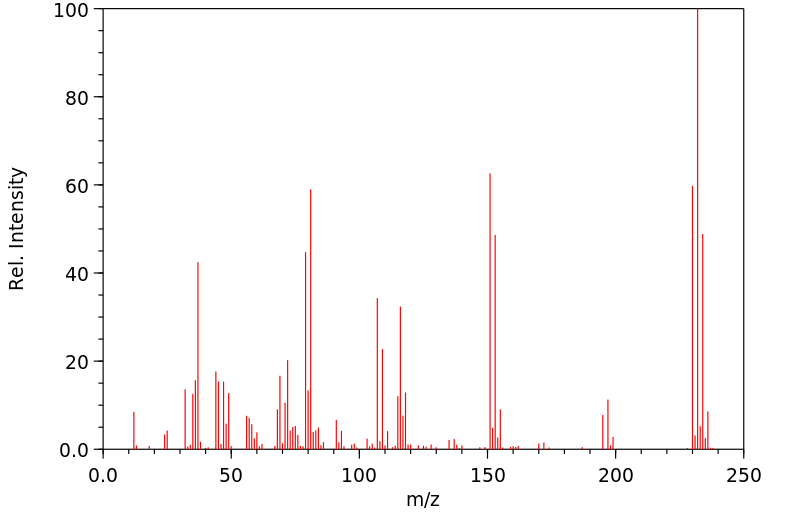
质谱MS
-
碳谱13CNMR
-
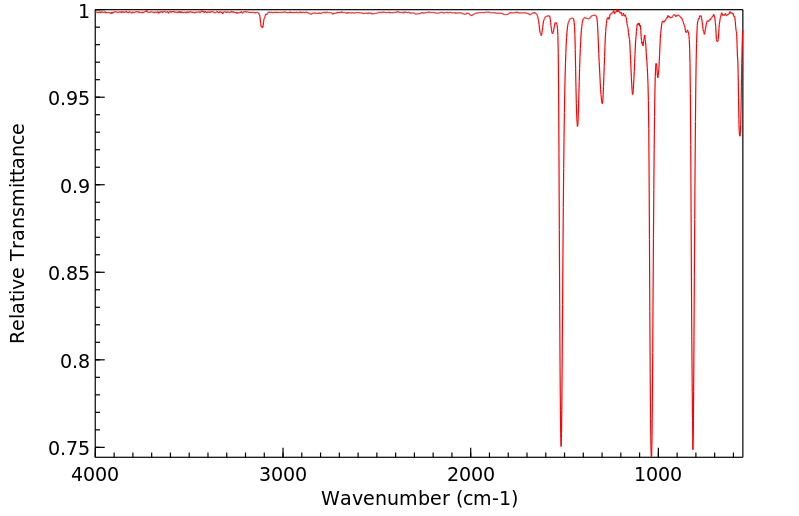
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿罗洛尔
阿替卡因
阿克兰酯
锡烷,(5-己基-2-噻吩基)三甲基-
邻氨基噻吩(2盐酸)
辛基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
辛基4,6-二溴噻吩并[3,4-b]噻吩-2-羧酸酯
辛基2-甲基异巴豆酸酯
血管紧张素IIAT2受体激动剂
葡聚糖凝胶LH-20
苯螨噻
苯并[c]噻吩-1-羧酸,5-溴-4,5,6,7-四氢-3-(甲硫基)-4-羰基-,乙基酯
苯并[b]噻吩-2-胺
苯并[b]噻吩-2-胺
苯基-[5-(4,4,5,5-四甲基-[1,3,2]二氧杂硼烷-2-基)-噻吩-2-基亚甲基]-胺
苯基-(5-氯噻吩-2-基)甲醇
苯乙酸,-α--[(1-羰基-2-丙烯-1-基)氨基]-
苯乙酰胺,3,5-二氨基-a-羟基-2,4,6-三碘-
苯乙脒,2,6-二氯-a-羟基-
腈氨噻唑
聚(3-丁基噻吩-2,5-二基),REGIOREGULAR
硝呋肼
硅烷,(3-己基-2,5-噻吩二基)二[三甲基-
硅噻菌胺
盐酸阿罗洛尔
盐酸阿罗洛尔
盐酸多佐胺
甲酮,[5-(1-环己烯-1-基)-4-(2-噻嗯基)-1H-吡咯-3-基]-2-噻嗯基-
甲基5-甲酰基-4-甲基-2-噻吩羧酸酯
甲基5-乙氧基-3-羟基-2-噻吩羧酸酯
甲基5-乙基-3-肼基-2-噻吩羧酸酯
甲基5-(氯甲酰基)-2-噻吩羧酸酯
甲基5-(氯乙酰基)-2-噻吩羧酸酯
甲基5-(氨基甲基)噻吩-2-羧酸酯
甲基5-(4-甲氧基苯基)-2-噻吩羧酸酯
甲基5-(4-甲基苯基)-2-噻吩羧酸酯
甲基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
甲基4-硝基-2-噻吩羧酸酯
甲基4-氰基-5-(4,6-二氨基吡啶-2-基)偶氮-3-甲基噻吩-2-羧酸酯
甲基4-氨基-5-(甲硫基)-2-噻吩羧酸酯
甲基4-{[(2E)-2-(4-氰基苯亚甲基)肼基]磺酰}噻吩-3-羧酸酯
甲基4-(氯甲酰基)-3-噻吩羧酸酯
甲基4-(氨基磺酰基氨基)-3-噻吩羧酸酯
甲基3-甲酰氨基-4-甲基-2-噻吩羧酸酯
甲基3-氨基-5-异丙基-2-噻吩羧酸酯
甲基3-氨基-5-(4-溴苯基)-2-噻吩羧酸酯
甲基3-氨基-4-苯基-5-(三氟甲基)-2-噻吩羧酸酯
甲基3-氨基-4-氰基-5-甲基-2-噻吩羧酸酯
甲基3-氨基-4-丙基-2-噻吩羧酸酯
甲基3-[[(4-甲氧基苯基)亚甲基氨基]氨基磺酰基]噻吩-2-羧酸酯