3-羟基四氢硫代吡喃 | 22072-19-1
中文名称
3-羟基四氢硫代吡喃
中文别名
——
英文名称
thiacyclohexan-3-ol
英文别名
3-hydroxytetrahydrothiopyran;thian-3-ol
CAS
22072-19-1
化学式
C5H10OS
mdl
——
分子量
118.2
InChiKey
ZHHOMIAGFRSWCU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:13-15 °C
-
沸点:152.48°C (estimate)
-
密度:1.1400
-
LogP:0.936 (est)
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:45.5
-
氢给体数:1
-
氢受体数:2
安全信息
-
安全说明:S24/25
-
海关编码:2934999090
SDS
| Name: | Tetrahydrothiopyran-3-ol 95% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 22072-19-1 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 22072-19-1 | Tetrahydrothiopyran-3-ol, 95% | 95% | 244-766-6 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid if cough or other symptoms appear.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Use only in a chemical fume hood.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower.
Exposure Limits CAS# 22072-19-1: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear a chemical apron.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: Clear
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.1480g/cm3
Molecular Formula: C5H10O5
Molecular Weight: 118.19
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Not available.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 22072-19-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Tetrahydrothiopyran-3-ol, 95% - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 22072-19-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 22072-19-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 22072-19-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-methoxythiacyclohexane 99797-73-6 C6H12OS 132.227
反应信息
-
作为反应物:描述:参考文献:名称:WO2008/147314摘要:公开号:
-
作为产物:描述:参考文献:名称:Pharmaceutically useful substituted thiacycloalkeno [3,2-b]pyridines,摘要:描述了一种新型的替代硫杂环烯[3,2-b]吡啶类化合物。这些化合物可作为钙通道拮抗剂,具有心血管、抗哮喘和抗支气管收缩活性。公开号:US04777167A1
文献信息
-
Iron-Catalyzed α-Alkylation of Ketones with Secondary Alcohols: Access to β-Disubstituted Carbonyl Compounds作者:Léo Bettoni、Sylvain Gaillard、Jean-Luc RenaudDOI:10.1021/acs.orglett.0c00549日期:2020.3.6borrowing hydrogen strategy has been applied in the synthesis of β-branched carbonyl compounds. Various secondary benzylic and aliphatic alcohols have been used as alkylating reagents under mild reaction conditions. The ketones have been isolated in good to excellent yield. Deuterium labeling experiments provide evidence that the alcohol is the hydride source in this reaction and that no reversible step or
-
2-alkylthiopenem derivatives
-
Herbicidal 1,2,4,6-thiatriazines申请人:Syngenta Investment Corporation公开号:US06362134B1公开(公告)日:2002-03-26Compounds of formula (I), in which R1, R2 and R3 are as defined in claim 1, are particularly suitable as herbicides.式(I)中的化合物,其中R1、R2和R3如权利要求1所定义,特别适用作为除草剂。
-
Novel compounds申请人:——公开号:US20040242573A1公开(公告)日:2004-12-02The invention relates to thiophene carboxanmides of formula (I). wherein A, R 1 , R 2 , R 3 , n and X are as defined in the specification, processes and intermediates used in their preparation, pharmaceutical compositions containing them and their use in therapy. 1本发明涉及公式(I)的噻吩羧酰胺,其中A,R1,R2,R3,n和X如规范中定义的,以及用于其制备的工艺和中间体,含有它们的制药组合物以及它们在治疗中的使用。
-
Thiophene carboxamide compounds as inhibitors of enzyme IKK-2申请人:AstraZeneca AB公开号:US07125896B2公开(公告)日:2006-10-24The invention relates to thiophene carboxanmides of formula (I), wherein A, R1, R2, R3, n and X are as defined in the specification, processes and intermediates used in their preparation, pharmaceutical compositions containing them and their use in therapy.本发明涉及公式(I)的噻吩羧酰胺,其中A,R1,R2,R3,n和X如规范中所定义,用于制备它们的过程和中间体,包含它们的制药组合物以及它们在治疗中的使用。
表征谱图
-
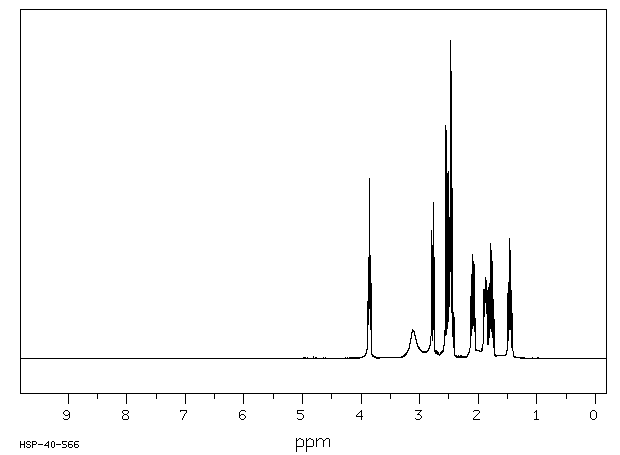
氢谱1HNMR
-
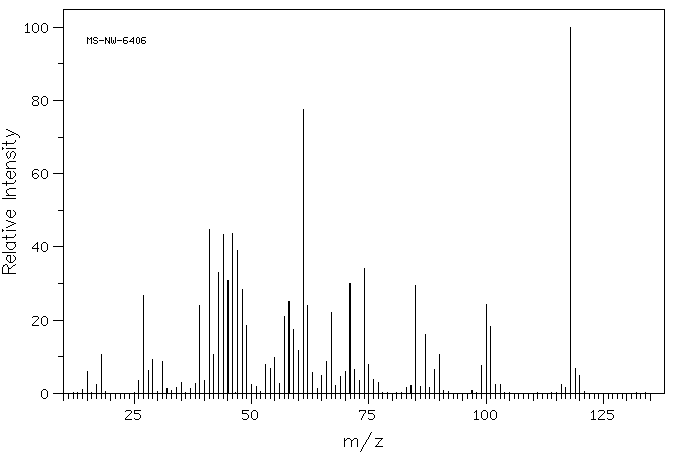
质谱MS
-
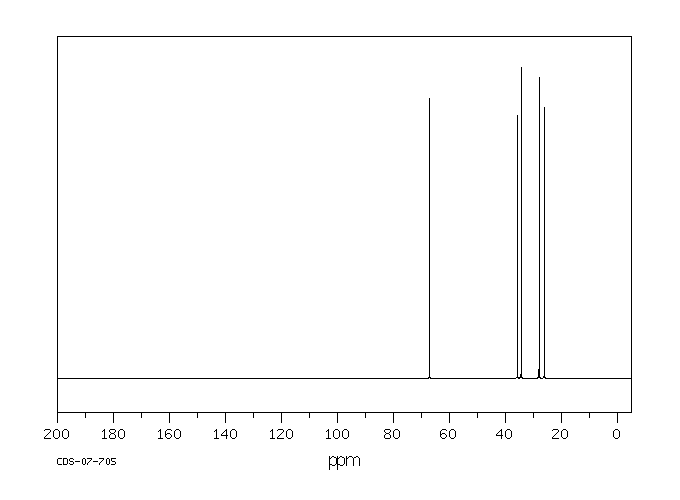
碳谱13CNMR
-
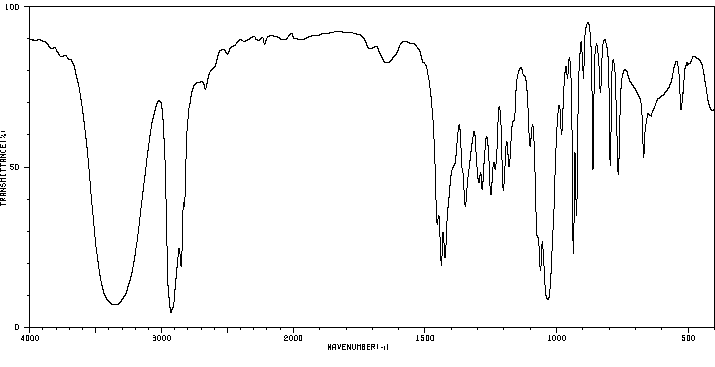
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿普卡林
硫化环戊烷
硫代环己酮
甲基硫酸四氢1-乙基-2-[1,2,3,4--1-(2-羟基乙基)-2,2,4-三甲基-6-喹啉基]苯[cd]吲哚正离子
甲基(1,1-二氧化四氢-2h-噻喃-4-基)醋酸盐
外-3-乙酰基-2-硫杂二环<2.2.2>辛-5-烯
四氢硫代吡喃-4-胺盐酸盐
四氢硫代吡喃-4-羰酰氯
四氢硫代吡喃-4-羧酸甲酯
四氢硫代吡喃-4-甲腈
四氢硫代吡喃-4-基甲醇
四氢硫代吡喃-3-甲醛
四氢噻喃-4-醇
四氢噻喃-4-酮肟
四氢噻喃-4-酮 1,1-二氧化物
四氢噻喃-4-酮
四氢噻喃-4-胺
四氢噻喃-4-肼双盐酸盐
四氢噻喃-4-甲醛
四氢-4H-硫代吡喃-4-酮 1-氧化物
四氢-4-氧代-2H-噻喃-3-甲酸甲酯
四氢-3-甲基-2H-噻喃
四氢-3-氧代-6H-噻喃-2-甲酸甲酯
四氢-2H-硫代吡喃-4-醇 1,1-二氧化物
四氢-2H-硫代吡喃-4-羧醛 1,1-二氧化物
四氢-2H-硫代吡喃-4-甲醇 1,1-二氧化物
四氢-2H-硫代吡喃-4-乙醛
四氢-2H-噻喃-4-羧酸甲酯1,1-二氧化物
四氢-2H-噻喃-4-甲酰肼
四氢-2H-噻喃-4-甲腈1,1-二氧化
四氢-2H-噻喃-3-醇1,1-二氧化物
四氢-2H-噻喃-3-羧酸1,1-二氧化物
四氢-(9ci)-2H-硫代吡喃-4-羧酸
噻-4-基甲胺
叔-丁基[(1S,2R)-1-苯甲基-2-羟基-3-[异丁基[(4-硝基苯基)磺酰]氨基]丙基]氨基甲酸酯
二氢-5,5-二甲基-2H-硫基吡喃-3(4H)-酮-1,1-二氧化物
二氢-2H-硫代吡喃-3(4h)-酮
二氢-2H-硫代吡喃-3(4H)-酮-1,1-二氧化物
乙酸四氢-2H-噻喃-2-基酯
三环己基乙基硼酸钠
n-[四氢-2H-硫代吡喃-4-基]氨基甲酸-1,1-二甲基乙酯
N-甲基四氢-2H-硫代吡喃-4-胺盐酸盐
N-甲基四氢-2H-噻喃-4-胺盐酸盐
N-甲基(四氢硫代吡喃-4-基)甲基胺
9-硫杂二环[3.3.1]壬烷-2,6-二酮
9-硫杂二环[3.3.1]壬烷
8-硫杂二环[3.2.1]辛烷-3-酮
8-硫杂-2,3-二氮杂螺[4.5]癸烷
8-乙烯基-7-硫杂-二环[4.2.0]辛烷
7-硫杂-2-氮杂螺[3.5]壬烷半草酸酯