癸二腈 | 4543-66-2
中文名称
癸二腈
中文别名
1,10-二氰癸烷;十二烷二腈;;十二烷二腈
英文名称
1,12-dodecanedinitrile
英文别名
1,10-dicyanodecane;dodecanedinitrile;1,10-Dicyandecan;Dodecandisaeuredinitril;dodecanedioic acid dinitrile
CAS
4543-66-2
化学式
C12H20N2
mdl
MFCD00013818
分子量
192.304
InChiKey
AVQYXBDAZWIFTO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:21°C
-
沸点:195°C 8mm
-
密度:0,898 g/cm3
-
闪点:202°C
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
稳定性/保质期:
在常温常压下保持稳定。
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:14
-
可旋转键数:9
-
环数:0.0
-
sp3杂化的碳原子比例:0.833
-
拓扑面积:47.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R20/21/22
-
RTECS号:JR2350000
-
海关编码:2926909090
-
危险品运输编号:3276
SDS
Dodecanedinitrile Revision number: 6
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Dodecanedinitrile
Revision number: 6
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Acute toxicity (Oral) Category 5
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements May be harmful if swallowed
Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Call a POISON CENTER or doctor/physician if you feel unwell.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Dodecanedinitrile
Percent: >98.0%(GC)
CAS Number: 4543-66-2
1,10-Dicyanodecane
Synonyms:
Chemical Formula: C12H20N2
Dodecanedinitrile
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Call a POISON CENTER or doctor/physician if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator, self-contained breathing apparatus(SCBA), supplied air respirator,
etc. Use respirators approved under appropriate government standards and follow
local and national regulations.
Impervious gloves.
Hand protection:
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Dodecanedinitrile
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Lump
White - Very pale yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:21°C (Freezing point)
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
420°C
Autoignition temperature:
Other data: The product is a liquid when ambient temperature is above melting point or due to
supercooling.
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
orl-rat LDLo:3400 mg/kg
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
RTECS Number: JR2350000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Dodecanedinitrile
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Dodecanedinitrile
Revision number: 6
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Acute toxicity (Oral) Category 5
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements May be harmful if swallowed
Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Call a POISON CENTER or doctor/physician if you feel unwell.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Dodecanedinitrile
Percent: >98.0%(GC)
CAS Number: 4543-66-2
1,10-Dicyanodecane
Synonyms:
Chemical Formula: C12H20N2
Dodecanedinitrile
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Call a POISON CENTER or doctor/physician if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator, self-contained breathing apparatus(SCBA), supplied air respirator,
etc. Use respirators approved under appropriate government standards and follow
local and national regulations.
Impervious gloves.
Hand protection:
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Dodecanedinitrile
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Lump
White - Very pale yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:21°C (Freezing point)
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
420°C
Autoignition temperature:
Other data: The product is a liquid when ambient temperature is above melting point or due to
supercooling.
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
orl-rat LDLo:3400 mg/kg
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
RTECS Number: JR2350000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Dodecanedinitrile
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Rasmussen,M. et al., Australian Journal of Chemistry, 1968, vol. 21, p. 2989 - 2999摘要:DOI:
-
作为产物:参考文献:名称:v. Braun, Chemische Berichte, 1909, vol. 42, p. 4548摘要:DOI:
文献信息
-
Synthesis of New Heterocyclic Fatty Compounds作者:Sandra Fürmeier、Jürgen O. MetzgerDOI:10.1002/ejoc.200390134日期:2003.3The terminal tetrazoles 1−5, the tetrazole analogues of the most important naturally occurring fatty acids, have been synthesized from fatty nitriles and completely characterized. The linear C12 bis(tetrazole) 6 was prepared and represents a valuable supplement to the previously known C2−C5 alkyl- and alkenyl-linked bis(tetrazoles). The tetrazoles 1−6 were converted into the respective 1,3,4-oxadiazoles末端四唑 1-5 是最重要的天然脂肪酸的四唑类似物,已由脂肪腈合成并已完全表征。制备了线性 C12 双(四唑)6,它是对先前已知的 C2-C5 烷基和烯基连接的双(四唑)的有价值的补充。通过在乙酸酐中加热,四唑 1-6 转化为相应的 1,3,4-恶二唑 13-17。还获得了三个双(恶二唑) 18-20。1,5-二取代四唑 12 是由 9(10)-氧代十八酸甲酯通过改进的施密特反应合成的。由顺式 9,10-环氧十八酸甲酯 (21) 制备了各种杂环,例如 4,5-二氢恶唑 22、恶唑烷 24、咪唑 26、恶唑 27 和咪唑啉硫酮 28。由于它们与天然存在的前列腺素的结构关系,化合物 12、22、24、26、27 和 28 应该作为高前列腺素类化合物而受到关注。(© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2003)
-
腈及其相应胺的制造方法申请人:中国石化扬子石油化工有限公司公开号:CN105001033B公开(公告)日:2018-06-22本发明涉及一种腈的制造方法,与现有技术相比,具有氨源用量显著降低、环境压力小、能耗低、生产成本低、腈产品的纯度和收率高等特点,并且能够获得结构更为复杂的腈。本发明还涉及由该腈制造相应胺的方法。
-
The Synthesis of α, ω-Disubstituted Higher Alkanes from α, α, α, ω-Tetrachloroalkanes作者:Kazuo Saotome、Hiroshi Komoto、Toshiaki YamazakiDOI:10.1246/bcsj.39.480日期:1966.3methods of the hydrolysis of α, α, α, ω-tetrachloroalkanes. The reactions were carried out in fuming nitric acid, with the addition of nitrogen dioxide during the reaction or in the presence of a small amount of phosphoric anhydride. α, ω-Dichloroalkanes were derived from the ω-chloroalkanoic acids by the Kolbe electrolytic synthesis. Various other novel α, ω-disubstituted alkanes with very long methylene
-
一种Ru配合物催化伯胺无受体脱氢生成腈的 方法申请人:四川大学公开号:CN111635334B公开(公告)日:2021-11-30
-
Ni-Catalyzed Reductive Homocoupling of Unactivated Alkyl Bromides at Room Temperature and Its Synthetic Application作者:Yu Peng、Long Luo、Chang-Song Yan、Jian-Jian Zhang、Ya-Wen WangDOI:10.1021/jo401936v日期:2013.11.1unactivated primary, secondary, and tertiary alkyl bromides is described. The catalytic system can be easily generated from air-stable and cheap materials and demonstrates broad functional group tolerance, thus allowing facile access to useful dimeric triterpene and lignan-like molecules. Moreover, the dimerization of tertiary bromide 6 efficiently establishes sterically hindered vicinal quaternary
表征谱图
-
氢谱1HNMR
-
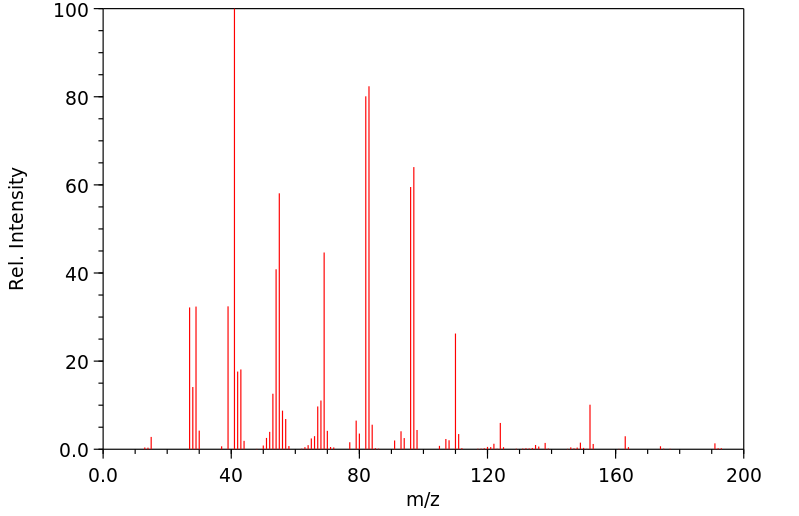
质谱MS
-
碳谱13CNMR
-
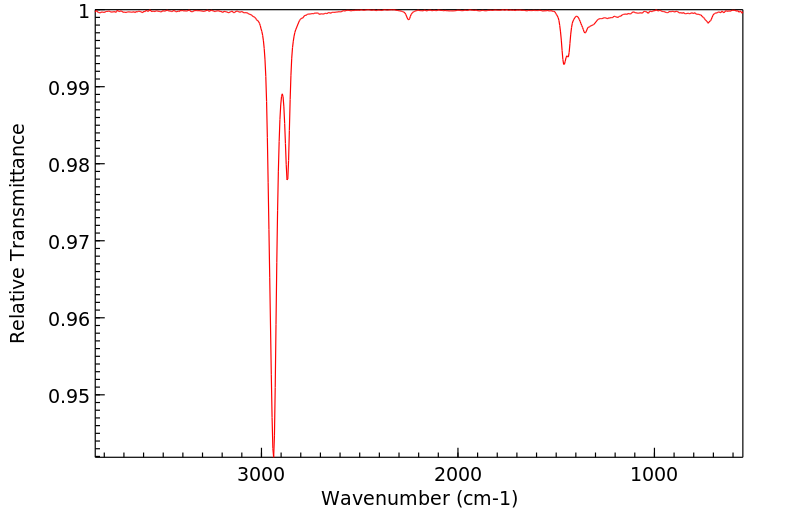
红外IR
-
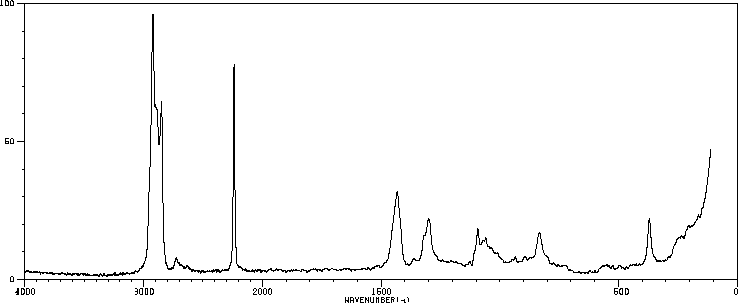
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷