2,2-二甲基-4,4-二氨基联苄 | 54628-21-6
中文名称
2,2-二甲基-4,4-二氨基联苄
中文别名
2,2'-二甲基-4,4'-二氨基联苄
英文名称
4,4'-diamino-2,2'-dimethylbibenzyl
英文别名
2,2'-dimethyl-4,4'-diaminobibenzyl;2,2'-dimethyl-bibenzyl-4,4'-diyldiamine;4,4'-ethylenedi-m-toluidine;4,4'-Ethylenedi-3-toluidine;4-[2-(4-amino-2-methylphenyl)ethyl]-3-methylaniline
CAS
54628-21-6
化学式
C16H20N2
mdl
——
分子量
240.348
InChiKey
ISESBQNCWCFFFR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:170 °C
-
沸点:411℃
-
密度:1.092
-
闪点:242℃
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:18
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:52
-
氢给体数:2
-
氢受体数:2
安全信息
-
海关编码:2921590090
SDS
Section I.Chemical Product and Company Identification
Chemical Name 4,4'-Diamino-2,2'-dimethylbibenzyl
Portland OR
Synonym 4,4'-Ethylenedi-m-toluidine
Chemical Formula [-CH2C6H3(NH2)CH3]2
CAS Number 22856-62-8
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
4,4'-Diamino-2,2'-dimethylbibenzyl 22856-62-8 Min. 95.0 (T) Not available. Not available.
Section III. Hazards Identification
Acute Health Effects Harmful if ingested or inhaled. Minimize exposure to this material. Severe overexposure can result in injury or death.
Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the
eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening,
or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when
handling this compound.
Chronic Health Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is
not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact
Check for and remove any contact lenses. DO NOT use an eye ointment. Flush eyes with running water for a minimum
of 15 minutes, occasionally lifting the upper and lower eyelids. Seek medical attention. Treat symptomatically and
supportively.
Skin Contact
After contact with skin, wash immediately with plenty of water. Gently and thoroughly wash the contaminated skin with
running water and non-abrasive soap. Be particularly careful to clean folds, crevices, creases and groin. Cover the
irritated skin with an emollient. Seek medical attention. Treat symptomatically and supportively. Wash any contaminated
clothing before reusing.
If the victim is not breathing, perform artificial respiration. Loosen tight clothing such as a collar, tie, belt or waistband. If
Inhalation
breathing is difficult, oxygen can be administered. Seek medical attention. Treat symptomatically and supportively.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt, or waistband. If the victim is not breathing, administer artificial respiration.
Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic material
was ingested; the absence of such signs, however, is not conclusive. Seek immediate medical attention and, if possible,
show the chemical label. Treat symptomatically and supportively.
Section V. Fire and Explosion Data
Not available.
Flammability Combustible. Auto-Ignition
Flash Points Flammable Limits Not available.
Not available.
Combustion Products
These products are toxic carbon oxides (CO, CO 2), nitrogen oxides (NO, NO2).
Fire Hazards
No specific information is available regarding the flammability of this compound in the presence of various materials.
Explosion Hazards Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
No additional information is available regarding the risks of explosion.
Fire Fighting Media
SMALL FIRE: Use DRY chemicals, CO 2, water spray or foam.
and Instructions LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
Continued on Next Page
4,4'-Diamino-2,2'-dimethylbibenzyl
Section VI. Accidental Release Measures
Spill Cleanup Harmful material. Irritating material.
Instructions In case of a spill and/or a leak, always shut off any sources of ignition, ventilate the area, and exercise caution. Use a
shovel to put the material into a convenient waste disposal container. Finish cleaning the spill by rinsing any
contaminated surfaces with copious amounts of water. Consult federal, state, and/or local authorities for assistance on
disposal.
Section VII. Handling and Storage
HARMFUL. IRRITANT. Keep away from heat and sources of ignition. Mechanical exhaust required. When not in use,
Handling and Storage
tightly seal the container and store in a dry, cool place. Avoid excessive heat and light. DO NOT ingest. DO NOT
Information
breathe dust. In case of insufficient ventilation, wear suitable respiratory equipment. If ingested, seek medical advice
immediately and show the container or the label. Treat symptomatically and supportively. Avoid contact with skin and
eyes.
Always store away from incompatible compounds such as oxidizing agents, acids.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below
recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to
airborne contaminants below the exposure limit.
Personal Protection Splash goggles. Lab coat. Dust respirator. Boots. Gloves. A MSHA/NIOSH approved respirator must be used to avoid
inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling
this product.
Exposure Limits
Not available.
Section IX. Physical and Chemical Properties
Physical state @ 20°C Solid. Solubility
Not available.
Not available.
Specific Gravity
Molecular Weight Partition Coefficient
240.35 Not available.
Boiling Point Vapor Pressure
Not available. Not available.
Melting Point 170 to 173°C (338 to 343.4°F) Vapor Density Not available.
Refractive Index Not available. Volatility Not available.
Critical Temperature Not available. Odor Not available.
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents, acids, chloroformates, acid chlorides, and acid anhydrides.
Section XI. Toxicological Information
RTECS Number Not available.
Routes of Exposure Eye contact. Inhalation. Ingestion. Skin contact.
Toxicity Data Not available.
CARCINOGENIC EFFECTS : Not available.
Chronic Toxic Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is not
known to aggravate existing medical conditions.
Acute Toxic Effects Harmful if ingested or inhaled. Minimize exposure to this material. Severe overexposure can result in injury or death.
Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the
eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening,
or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when
handling this compound.
Continued on Next Page
4,4'-Diamino-2,2'-dimethylbibenzyl
Section XII. Ecological Information
Ecotoxicity Not available.
Environmental Fate Not available.
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local or regional authorities. You may be able to dissolve or mix material with
Waste Disposal
a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state, and local regulations when disposing of this substance.
Section XIV. Transport Information
DOT Classification Not a DOT controlled material (United States).
PIN Number Not applicable.
Proper Shipping Name
Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification WHMIS CLASS D-2A: Material causing other toxic effects (VERY TOXIC).
(Canada)
EINECS Number (EEC) 245-268-1
EEC Risk Statements R36/37/38- Irritating to eyes, respiratory system and skin.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲基-1-[2-(2-甲基-4-硝基苯基)乙基]-4-硝基苯 2,2'-dimethyl-4,4'-dinitrobibenzyl 87517-98-4 C16H16N2O4 300.314
反应信息
-
作为反应物:描述:2,2-二甲基-4,4-二氨基联苄 在 吡啶 、 potassium carbonate 、 N,N-二甲基甲酰胺 作用下, 生成 11,18-dimethyl-1,8-diaza-[8.2]paracyclophane参考文献:名称:Heterocycles Containing p-Phenylene Units. III. Substituted Amines摘要:DOI:10.1021/ja01118a514
-
作为产物:描述:4-硝基邻二甲苯 在 三乙醇胺 、 tetrafluoroborate 1-hexyl-1,8-diazabicyclo(5,4,0)undec-7-ene 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 生成 2,2-二甲基-4,4-二氨基联苄参考文献:名称:一种二氨基二苯乙烷化合物的合成方法摘要:本发明公开一种二氨基二苯乙烷化合物的合成方法,涉及化合物制备技术领域;向离子液体催化剂、硝基邻二甲苯中加入碱性溶液,配制成硝基邻二甲苯溶液,硝基邻二甲苯溶液在连续通氧气的作用下进行偶联反应,中和至中性,若碱性溶液中溶剂与水互溶,则过滤、水洗,得到二甲基二硝基二苯乙烷固体,将二甲基二硝基二苯乙烷固体制成二甲基二硝基二苯乙烷溶液,若碱性溶液中溶剂与水不互溶,则分层,得到油层为二甲基二硝基二苯乙烷溶液,将二甲基二硝基二苯乙烷溶液在连续通氢气的作用下进行加氢反应,反应液分层后,油层进行蒸馏,得到二甲基二氨基二苯乙烷。公开号:CN110862323A
文献信息
-
NOVEL COMPOUND HAVING MULTIMER STRUCTURE OF XANTHENE DERIVATIVE, COLORING COMPOSITION, INK FOR INKJET RECORDING, METHOD OF INKJET RECORDING, COLOR FILTER, AND COLOR TONER申请人:FUJIFILM CORPORATION公开号:US20140176653A1公开(公告)日:2014-06-26There is provided a compound represented by formula (1): in formula (1), L represents a divalent to tetravalent linking group; D represents a residue obtained by removing 1 to 5 hydrogen atoms from a compound represented by formula (2); m represents an integer of 1 to 10, however, each L may be the same with or different from every other L; n represents an integer of 2 to 10, however, each D may be the same with or different from every other D; and in formula (2), each of R 4 to R 24 independently represents a hydrogen atom or a substituent, provided that formula (2) has at least one or more ionic hydrophilic groups.提供了一种由公式(1)表示的化合物:在公式(1)中,L代表二价到四价的连接基团;D代表通过从由公式(2)表示的化合物中去除1到5个氢原子获得的残基;m代表1到10的整数,但是,每个L可以相同也可以不同于其他L;n代表2到10的整数,但是,每个D可以相同也可以不同于其他D;在公式(2)中,R4到R24中的每一个独立地代表一个氢原子或一个取代基,前提是公式(2)至少有一个或更多的离子亲水基团。
-
PHOTOREACTIVE COMPOUNDS申请人:Lincker Frederic公开号:US20140192305A1公开(公告)日:2014-07-10The present invention relates to photoreactive compounds that are particularly useful in materials for the alignment of liquid crystals.本发明涉及光反应性化合物,特别适用于液晶对准材料的制作。
-
一种二苯基乙烷二异氰酸酯及其制备方法
-
[EN] PHOTOACTIVE MATERIALS<br/>[FR] MATERIAUX PHOTOACTIFS申请人:ROLIC AG公开号:WO2004013086A1公开(公告)日:2004-02-12Diamine compounds, which in particular are useful as precursors for the production of liquid crystal alignment layers, represented by general formula (I), wherein A1 represents an organic group of 1 to 40 carbon atoms; A2 represents a hydrogen atom or an organic group of 1 to 40 carbon atoms.二胺化合物,特别是作为液晶取向层生产的前体物而有用,由一般式(I)代表,其中A1代表1至40个碳原子的有机基团;A2代表氢原子或1至40个碳原子的有机基团。
-
Compound having silsesquioxane skeleton and its polymer申请人:Inagaki Jyun-ichi公开号:US20050009982A1公开(公告)日:2005-01-13The present invention relates to a compound represented by Formula (1) and a polymer obtained using the compound: wherein R 1 is phenyl which may have substituents, Q 1 is hydrogen, halogen, alkyl having 1 to 10 carbon atoms, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclohexenyl or phenyl in which optional hydrogen may be replaced by halogen or alkyl having 1 to 5 carbon atoms, and Q 2 is a group represented by Formula (2) wherein the code < represents a bonding point with silicon, l, m, n and p are independently 0, 1, 2 or 3, A 1 to A 4 are independently a single bond, 1,4-cyclohexylene, 1,4-cyclohexenylene, a condensed ring group having 6 to 10 carbon atoms which is a divalent group, or 1,4-phenylene, Z 0 to Z 3 are independently a single bond, —CH═CR—, —C≡C—, —COO—, —OCO—, or alkylene having 1 to 20 carbon atoms, and Z 4 is a single bond, —CH═CH—, —C≡C—, —COO—, —OCO—, or alkylene having 1 to 20 carbon atoms. And Y 1 in Formula (1) is the group defined in Claim 1.本发明涉及一种由式(1)表示的化合物和使用该化合物获得的聚合物:其中R1是苯基,可能具有取代基;Q1是氢、卤素、具有1至10个碳原子的烷基、环丙基、环丁基、环戊基、环己基、环己烯基或苯基,其中可选的氢原子可被卤素或具有1至5个碳原子的烷基取代;Q2是由式(2)表示的基团,其中代码<表示与硅的连接点,l、m、n和p独立地为0、1、2或3,A1至A4独立地为单键、1,4-环己亚基、1,4-环己烯亚基、具有6至10个碳原子的缩合环基团,为二价基团,或1,4-苯亚基,Z0至Z3独立地为单键、—CH═CR—、—C≡C—、—COO—、—OCO—或具有1至20个碳原子的烷基,Z4为单键、—CH═CH—、—C≡C—、—COO—、—OCO—或具有1至20个碳原子的烷基。式(1)中的Y1是权利要求1中定义的基团。
表征谱图
-
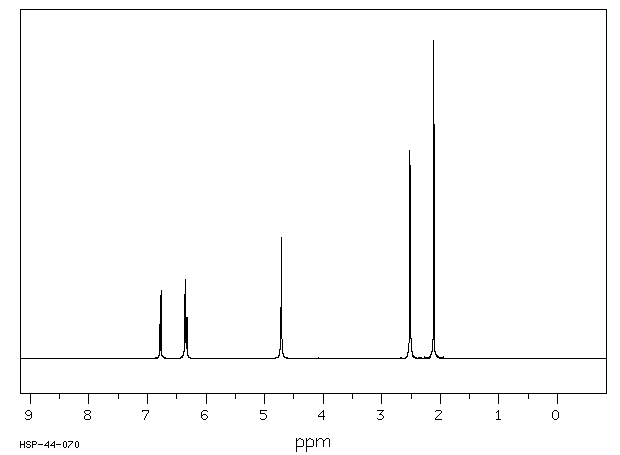
氢谱1HNMR
-
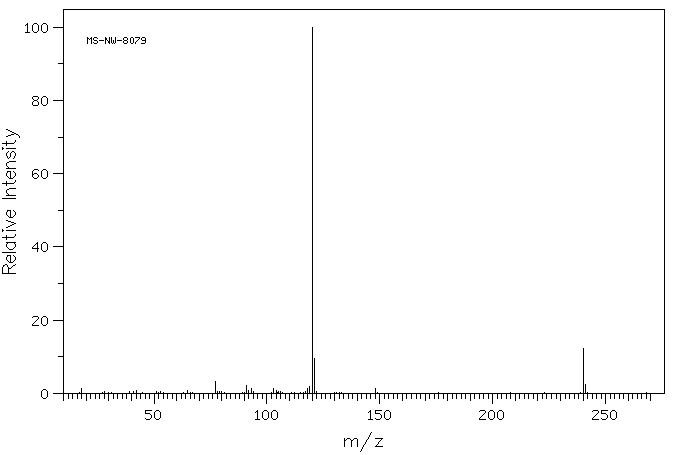
质谱MS
-
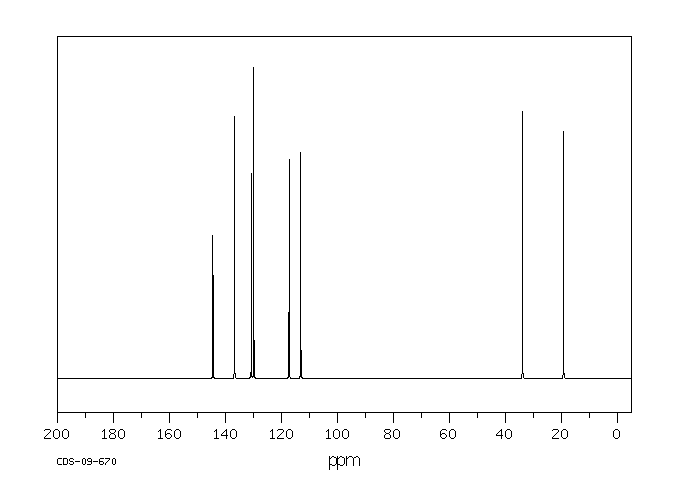
碳谱13CNMR
-
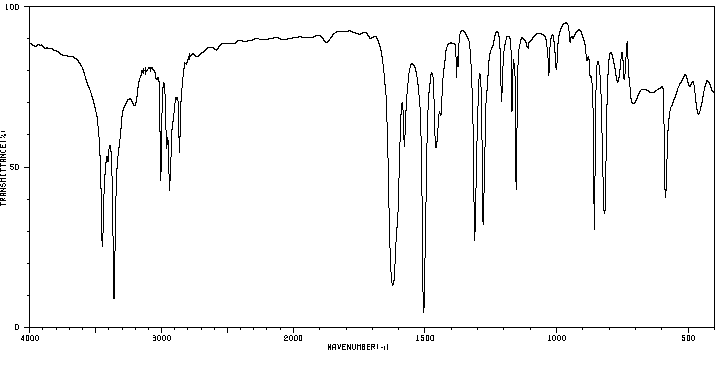
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E,Z)-他莫昔芬N-β-D-葡糖醛酸
(E/Z)-他莫昔芬-d5
(4S,5R)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S,5R,5''R)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(4R,5S)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4R,4''R,5S,5''S)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(1R,2R)-2-(二苯基膦基)-1,2-二苯基乙胺
鼓槌石斛素
黄子囊素
高黄绿酸
顺式白藜芦醇三甲醚
顺式白藜芦醇
顺式己烯雌酚
顺式-白藜芦醇3-O-beta-D-葡糖苷酸
顺式-桑皮苷A
顺式-曲札芪苷
顺式-二苯乙烯
顺式-beta-羟基他莫昔芬
顺式-a-羟基他莫昔芬
顺式-3,4',5-三甲氧基-3'-羟基二苯乙烯
顺式-1-(3-甲基-2-萘基)-2-(2-萘基)乙烯
顺式-1,2-双(三甲基硅氧基)-1,2-双(4-溴苯基)环丙烷
顺式-1,2-二苯基环丁烷
顺-均二苯乙烯硼酸二乙醇胺酯
顺-4-硝基二苯乙烯
顺-1-异丙基-2,3-二苯基氮丙啶
非洲李(PRUNUSAFRICANA)树皮提取物
阿非昔芬
阿里可拉唑
阿那曲唑二聚体
阿托伐他汀环氧四氢呋喃
阿托伐他汀环氧乙烷杂质
阿托伐他汀环(氟苯基)钠盐杂质
阿托伐他汀环(氟苯基)烯丙基酯
阿托伐他汀杂质D
阿托伐他汀杂质94
阿托伐他汀杂质7
阿托伐他汀杂质5
阿托伐他汀内酰胺钠盐杂质
阿托伐他汀中间体M4
阿奈库碘铵
锌(II)(苯甲醛)(四苯基卟啉)
银松素
铜酸盐(5-),[m-[2-[2-[1-[4-[2-[4-[[4-[[4-[2-[4-[4-[2-[2-(羧基-kO)苯基]二氮烯基-kN1]-4,5-二氢-3-甲基-5-(羰基-kO)-1H-吡唑-1-基]-2-硫代苯基]乙烯基]-3-硫代苯基]氨基]-6-(苯基氨基)-1,3,5-三嗪-2-基]氨基]-2-硫代苯基]乙烯基]-3-硫代
铒(III) 离子载体 I
铀,二(二苯基甲酮)四碘-
钾钠2,2'-[(E)-1,2-乙烯二基]二[5-({4-苯胺基-6-[(2-羟基乙基)氨基]-1,3,5-三嗪-2-基}氨基)苯磺酸酯](1:1:1)
钠{4-[氧代(苯基)乙酰基]苯基}甲烷磺酸酯
钠;[2-甲氧基-5-[2-(3,4,5-三甲氧基苯基)乙基]苯基]硫酸盐
钠4-氨基二苯乙烯-2-磺酸酯