苯并[b]噻吩-3-基乙酸酯 | 24434-82-0
中文名称
苯并[b]噻吩-3-基乙酸酯
中文别名
——
英文名称
benzo[b]thiophen-3-yl acetate
英文别名
2-Acetoxy-benzothiophen;(benzo[b]thiophen-3-yl) acetate;3-acetoxybenzothiophene;3-acetoxy-thianaphtene;3-acetoxy-benzo[b]thiophene;acetic acid benzo[b]thiophen-3-yl ester;1-benzothiophen-3-yl acetate
CAS
24434-82-0
化学式
C10H8O2S
mdl
——
分子量
192.238
InChiKey
IFMOFXZTHWSMEA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:165 °C(Press: 18 Torr)
-
密度:1.2580 g/cm3(Temp: 20.4 °C)
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:13
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:54.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2934999090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-羟基苯并噻吩 3-hydroxybenzothiophene 520-72-9 C8H6OS 150.201
反应信息
-
作为反应物:描述:苯并[b]噻吩-3-基乙酸酯 在 sodium hydride 、 lithium diisopropyl amide 作用下, 以 四氢呋喃 为溶剂, 生成 2-hydroxy-2,3-dihydrobenzo[4,5]thieno[3,2-b]pyran-4-one参考文献:名称:定向金属化在合成中的应用。第7部分:适当官能化的苯并[ b ]噻吩的合成作为苯并噻吩并吡喃酮合成中的关键中间体摘要:一锅合成由邻甲基硫烷基芳基N,N-二乙基酰胺与1-(3-羟基苯并[ b ]噻吩-2-基)乙酮和1合成(3-羟基苯并[ b ]噻吩-2-基)芳基甲酮描述了经由阴离子邻-Fries重排的-(3-羟基苯并[ b ]噻吩-2-基)丙-1-酮。羟基酮用作苯并噻吩并吡喃酮合成中的关键中间体。DOI:10.1016/j.tetlet.2005.01.038
-
作为产物:描述:N,N-二乙基苯甲酰胺 在 四甲基乙二胺 、 仲丁基锂 、 sodium hydride 、 lithium diisopropyl amide 作用下, 以 四氢呋喃 为溶剂, 反应 10.0h, 生成 苯并[b]噻吩-3-基乙酸酯参考文献:名称:Application of directed metalation in synthesis. Part 8: Interesting example of chemoselectivity in the synthesis of thioaurones and hydroxy ketones and a novel anionic ortho-Fries rearrangement used as a tool in the synthesis of thienopyranones and thiafluorenones摘要:Chemoselective synthesis of thioaurones or 3-hydroxy benzo[b]thiophen-2-aryl ketones, 1-hydroxy naphtho[2,1-b]thiophen-2aryl ketones and chalcones from N,N-diethyl-ortho-methyl sulfanyl aryl amides were described. (Benzo[b]thiophen-2-yl) alkylates and (naphtho[2, 1-b]thiophen-2-yl) alkylates undergo a novel anionic ortho-Fries rearrangement leading to (3-hydroxy benzo[b]thiophen-2-yl) and (1-hydroxy naphtho[2, 1 -b]thiophen-2-yl) alkyl ketones. The hydroxy ketones were used as intermediates in the synthesis of wide range of benzothienopyranones and thiafluorenones. (c) 2005 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2005.07.050
文献信息
-
Synthesis and studies of tris-indolobenzenes and related compounds作者:Jan Bergman、Nils EklundDOI:10.1016/0040-4020(80)85060-5日期:1980.1The unsymmetrical N,N,N-trimethyl tris-indolobenzene 3 has been synthesized by several routes, including cyclotrimerization of the O-acetate of indoxyl. This condensation involves a 3→2 rearrangement of the precursor formed in situ. Similar Wagner-Meerwein rearrangements were also prevalent in LAH reductions of some 3,3-diindolyl oxindoles.
-
[EN] FUSED-ARYL AND HETEROARYL DERIVATIVES AND METHODS OF THEIR USE<br/>[FR] DERIVES HETEROARYLE ET ARYLE FUSIONNE ET METHODES D'UTILISATION ASSOCIEES申请人:WYETH CORP公开号:WO2005037283A1公开(公告)日:2005-04-28The present invention is directed to fused-aryl and heteroaryl derivatives of formula I, compositions containing these derivatives, and methods of their use for the prevention and treatment of conditions ameliorated by monoamine reuptake including, inter alia, vasomotor symptoms (VMS), sexual dysfunction, gastrointestinal and genitourinary disorders, chronic fatigue syndrome, fibromylagia syndrome, nervous system disorders, and combinations thereof, particularly those conditions selected from the group consisting of major depressive disorder, vasomotor symptoms, stress and urge urinary incontinence, fibrornyalgia, pain, diabetic neuropathy, and combinations thereof. FORMULA
-
[EN] 1- 2' (1, 4'-BIPERIDIN-1'-YL)-1- (PHENYL) -ETHYL CYCLOHEXANOL DERIVATIVES AS MONOAMINE REUPTAKE MODULATORS FOR THE TREATMENT OF VISOMOTOR SYMPTOMS<br/>[FR] DERIVES DE 1- 2' (1, 4'-BIPERIDIN-1'-YL)-1- (PHENYL) ETHYL CYCLOHEXANOL UTILES COMME MODULATEURS DU RECAPTAGE DE MONOAMINE DANS LE TRAITEMENT DES SYMPTOMES VASOMOTEURS申请人:WYETH CORP公开号:WO2005037279A1公开(公告)日:2005-04-28The present invention is directed to substituted N-heterocycle derivatives of formula (I), compositions containing these derivatives, and their use in the manufacture of a medicament for the prevention and treatment of conditions ameliorated by monoamine reuptake including, inter alia, vasomotor symptoms (VMS), sexual dysfunction, gastrointestinal and genitourinary disorders, chronic fatigue syndrome, fibromylagia syndrome, nervous system disorders, and combinations thereof, particularly those conditions selected from the group consisting of major depressive disorder, vasomotor symptoms, stress and urge urinary incontinence, fibromyalgia, pain, diabetic neuropathy, and combinations thereof wherein : A is phenyl, naphthyl, thiophenyl, pyridinyl, furanyl, benzofuranyl, isobenzofuranyl, xanthenyl, pyrrolyl, indolizinyl, isoindolyl, indolyl, or benzothiophenyl, where 1 to 3 carbon atoms of said A is optionally replaced with a nitrogen atom; U is N or O; W is H or OR8; the other substituents are defined in the claims.本发明涉及式(I)的取代N-杂环衍生物,含有这些衍生物的组合物,以及它们在制造用于预防和治疗由单胺再摄取改善的病况的药物中的应用,包括但不限于血管运动症状(VMS)、性功能障碍、胃肠道和泌尿系统疾病、慢性疲劳综合征、纤维肌痛综合征、神经系统疾病以及其中的组合,特别是从主要抑郁障碍、血管运动症状、压力和急迫性尿失禁、纤维肌痛、疼痛、糖尿病性神经病变以及其中的组合中选择的那些病况,其中:A是苯基、萘基、噻吩基、吡啶基、呋喃基、苯并呋喃基、异苯并呋喃基、黄色素基、吡咯基、吲哚啉基、异吲哚基、吲哚基或苯并噻吩基,其中所述A的1至3个碳原子可选择地被氮原子取代;U是N或O;W是H或OR8;其他取代基在权利要求中定义。
-
[EN] SUBSTITUTED ARYL CYCLOALKANOL DERIVATIVES AND METHODS OF THEIR USE<br/>[FR] DERIVES DE CYCLOALCANOYL ARYLE SUBSTITUES ET PROCEDES D'UTILISATION DE CEUX-CI申请人:WYETH CORP公开号:WO2005037809A1公开(公告)日:2005-04-28The present invention is directed to substituted aryl cycloalkanoyl derivatives, compositions containing these derivatives, and methods of their use for the prevention and treatment of conditions ameliorated by monoamine reuptake including, inter alia, vasomotor symptoms (VMS), sexual dysfunction, gastrointestinal and genitourinary disorders, chronic fatigue syndrome, fibromylagia syndrome, nervous system disorders, and combinations thereof, particularly those conditions selected from the group consisting of major depressive disorder, vasomotor symptoms, stress and urge urinary incontinence, fibromyalgia, pain, diabetic neuropathy, and combinations thereof.
-
[EN] ARYLALKYL- AND CYCLOALKYLALKYL-PIPERAZINE DERIVATIVES AND METHODS OF THEIR USE<br/>[FR] DERIVES D'ARYLALKYL-PIPERAZINE ET DE CYCLOALKYLALKYL-PIPERAZINE ET METHODES D'UTILISATION ASSOCIEES申请人:WYETH CORP公开号:WO2005037807A1公开(公告)日:2005-04-28The present invention is directed to arylalkyl- and cycloalkylalkyl-piperazine derivatives, compositions containing these derivatives, and methods of their use for the prevention and treatment of conditions ameliorated by monoamine reuptake including, inter alia, vasomotor symptoms (VMS), sexual dysfunction, gastrointestinal and genitourinary disorders, chronic fatigue syndrome, fibromylagia syndrome, nervous system disorders, and combinations thereof, particularly those conditions selected from the group consisting of major depressive disorder, vasomotor symptoms, stress and urge urinary incontinence, fibromyalgia, pain, diabetic neuropathy, and combinations thereof.
表征谱图
-
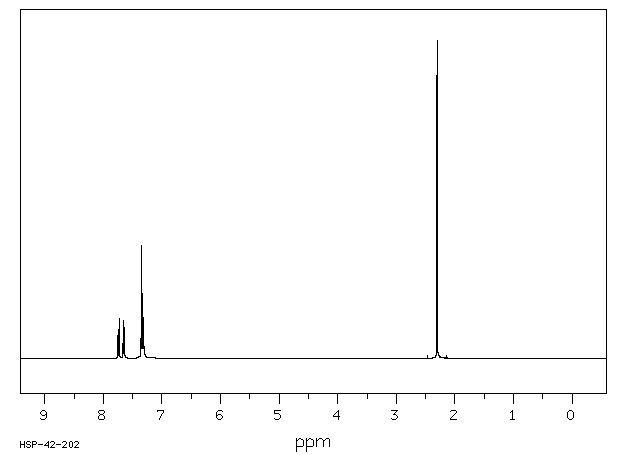
氢谱1HNMR
-
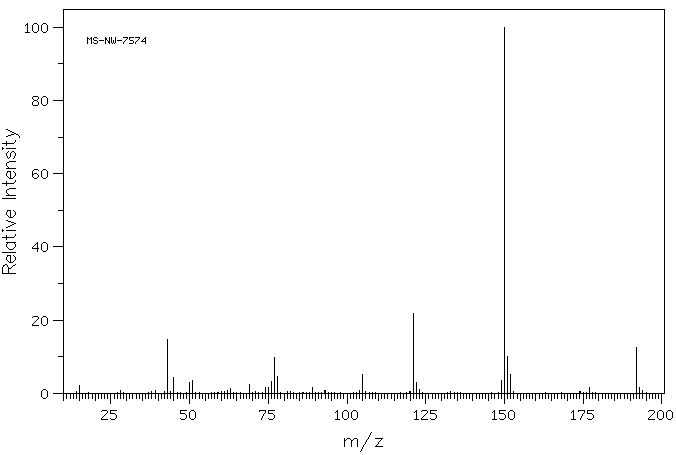
质谱MS
-
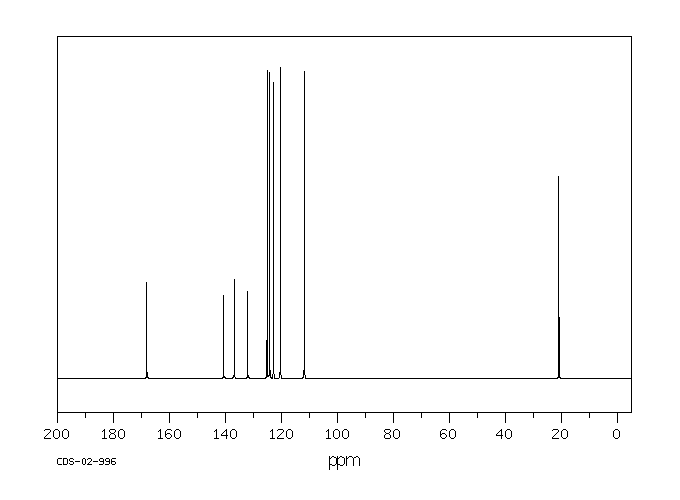
碳谱13CNMR
-
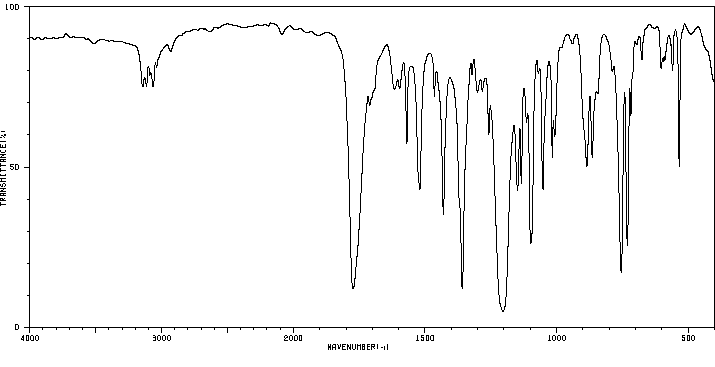
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐留通钠
齐留通相关物质A
齐留通亚砜
齐留通-d4
齐留通
雷洛昔芬杂质
邻联甲苯胺砜
试剂4,8-Bis(3,5-dioctyl-2-thienyl)-2,6-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[1,2-b:4,5-b']dithiophene
试剂1,1'-[4,8-Bis[4-(2-ethylhexyl)-3,5-difluorophenyl]benzo[1,2-b:4,5-b']dithiophene-2,6-diyl]bis[1,1,1-trimethylstannane]
苯并噻吩-7-醇
苯并噻吩-4-硼酸频哪醇酯
苯并噻吩-3-羧酸甲酯
苯并噻吩-3-硼酸
苯并噻吩-2-羰酰氯
苯并噻吩-2-羧酸肼
苯并噻吩-2-羧酸
苯并噻吩-2-硼酸
苯并噻吩-2-氨基甲酸叔丁酯
苯并噻吩
苯并[c]噻吩
苯并[b]噻吩-7-胺
苯并[b]噻吩-7-羧酸乙酯
苯并[b]噻吩-7-甲醛
苯并[b]噻吩-7-甲腈
苯并[b]噻吩-6-醇
苯并[b]噻吩-6-胺
苯并[b]噻吩-6-羧酸乙酯
苯并[b]噻吩-6-羧酸
苯并[b]噻吩-6-甲腈
苯并[b]噻吩-5-甲腈,2-甲酰基-
苯并[b]噻吩-5-甲磺酰氯
苯并[b]噻吩-4-羧酸甲酯
苯并[b]噻吩-4-羧酸
苯并[b]噻吩-4-甲醛
苯并[b]噻吩-4-甲腈
苯并[b]噻吩-4-基甲醇
苯并[b]噻吩-3-胺盐酸盐
苯并[b]噻吩-3-胺
苯并[b]噻吩-3-羧酸-(2-二烯丙基氨基乙酯)
苯并[b]噻吩-3-硼酸频哪酯
苯并[b]噻吩-3-甲醛肟
苯并[b]噻吩-3-甲酰胺
苯并[b]噻吩-3-基乙酸酯
苯并[b]噻吩-3-乙酸
苯并[b]噻吩-3-乙酰氯
苯并[b]噻吩-3-乙腈
苯并[b]噻吩-2-胺盐酸盐
苯并[b]噻吩-2-羧酸6-氨基-3-氯-甲酯
苯并[b]噻吩-2-羧酸,5-氯-3-(1-甲基乙氧基)-
苯并[b]噻吩-2-羧酸,3-羟基-5-甲氧基-,甲基酯