5,7-二甲基色酮-3-甲醛 | 62484-76-8
中文名称
5,7-二甲基色酮-3-甲醛
中文别名
5,7-二甲基色酮;5,7-二甲基-4-氧代-4H-亚甲基-3-甲醛
英文名称
5,7-dimethyl-4-oxo-4H-chromene-3-carbaldehyde
英文别名
5,7-Dimethyl-4-oxo-4H-chromene-3-carbaldehyde;5,7-dimethyl-4-oxochromene-3-carbaldehyde
CAS
62484-76-8
化学式
C12H10O3
mdl
MFCD00051509
分子量
202.21
InChiKey
MVKCOCQJKGTQAM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:145-147°C
-
沸点:362.7±42.0 °C(Predicted)
-
密度:1.311±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:15
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
安全说明:S22,S24/25
-
海关编码:2932999099
-
储存条件:存储条件:2-8°C,密封于干燥处。
SDS
反应信息
-
作为反应物:描述:5,7-二甲基色酮-3-甲醛 在 四氯苯醌 、 对甲苯磺酸 作用下, 以 xylene 、 苯 为溶剂, 生成 1,3-dimethyl-benzo[b]chromeno[3,2-f][1,4]thiazepin-13-one参考文献:名称:Reactions of Formylchromone Derivatives; 31. A Facile Synthesis of Fused Benzopyrano-benzothiazepinones, -benzoxazepinones, and -benzodiazepinones摘要:DOI:10.1055/s-1979-28669
文献信息
-
An environmentally benign cascade reaction of chromone-3-carboxaldehydes with ethyl 2-(pyridine-2-yl)acetate derivatives for highly site-selective synthesis of quinolizines and quinolizinium salts in water作者:Li Chen、Rong Huang、Kun Li、Xing-Han Yun、Chang-Long Yang、Sheng-Jiao YanDOI:10.1039/d0gc02460k日期:——quinolizinium salts (4) from chromone-3-carboxaldehydes 1 with ethyl 2-(pyridine-2-yl)acetate derivatives 2via an unprecedented cascade reaction in water was constructed. As a result, functionalized quinolizines (3) bearing a chromone skeleton were prepared by simple reflux of the mixture of chromone-3-carboxaldehydes with ethyl 2-(pyridine-2-yl)acetate derivatives in water. Quinolizinium salts (4) were
-
Synthesis and Screening of Fluoro Substituted Pyrazolyl Benzoxazoles作者:R. K Jadhav、A. B Nikumbh、B. K KaraleDOI:10.13005/ojc/310242日期:2015.6.20A series of 3-Formylchromone 1 was reacted with 1-(4-(4-fluorophenyl)thiazol-2-yl)hydrazine 2 to get (1-(4-(4-fluorophenyl)thiazol-2-yl)-1H-pyrazol-4-yl)(2-hydroxyphenyl) methanone 3 which on reaction with hydroxylamine hydrochloride given methanone oxime 4 and 4 on treatment with POCl3 formed 2-(1-(4-(4-fluorophenyl)thiazol-2-yl)-1H-pyrazol-4-yl)benzo[d]oxazole 5. The structures of synthesized compounds
-
Indolinone combinatorial libraries and related products and methods for the treatment of disease申请人:Tang Cho Peng公开号:US20050197382A1公开(公告)日:2005-09-08The present invention relates to organic molecules capable of modulating, regulating and/or inhibiting protein kinase signal transduction. Such compounds are useful for the treatment of diseases related to unregulated protein kinase signal transduction, including cell proliferative diseases such as cancer, atherosclerosis, arthritis and restenosis and metabolic diseases such as diabetes. The present invention features indolinone compounds that potently inhibit protein kinases and related products and methods. Inhibitors specific to the FLK protein kinase can be obtained by adding chemical substituents to the 3-[(indole-3-yl)methylene]-2-indolinone, in particular at the 1′ position of the indole ring. Indolinone compounds that specifically inhibit the FLK and platelet derived growth factor protein kinases can harbor a tetrahydroindole or cyclopentano-b-pyrrol moiety. Indolinone compounds that are modified with substituents, particularly at the 5 position of the oxindole ring, can effectively activate protein kinases. This invention also features novel hydrosoluble indolinone compounds that are tyrosine kinase inhibitors and related products and methods.本发明涉及有机分子,能够调节、调控和/或抑制蛋白激酶信号转导。这些化合物对于治疗与不受调节的蛋白激酶信号转导相关的疾病非常有用,包括细胞增殖性疾病,如癌症、动脉粥样硬化、关节炎、再狭窄和代谢性疾病,如糖尿病。本发明涉及具有强效抑制蛋白激酶和相关产品以及方法的吲哚酮化合物。特定于FLK蛋白激酶的抑制剂可以通过在吲哚环的1'位置添加化学取代基来获得3-[(吲哚-3-基)亚甲基]-2-吲哚酮。特异性抑制FLK和血小板衍生生长因子蛋白激酶的吲哚酮化合物可以具有四氢吲哚或环戊烯基-β-吡咯基。在氧吲哚环的5位取代基的修饰下,吲哚酮化合物可以有效激活蛋白激酶。本发明还涉及新型水溶性吲哚酮化合物,它们是酪氨酸激酶抑制剂和相关产品和方法。
-
PYRROL-1-YL BENZOIC ACID DERIVATIVES USEFUL AS MYC INHIBITORS申请人:DANA-FARBER CANCER INSTITUTE, INC.公开号:US20150291521A1公开(公告)日:2015-10-15The present invention provides compounds of Formula (I-A), (I-B), and (I-C), pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof. Compounds of the present invention are useful for inhibiting Myc (e.g., c-Myc) activity. The present invention further provides methods of using the compounds described herein for treating Myc-mediated disorders (e.g., cancer and other proliferative diseases). The present invention also provides assays for identifying Myc inhibitors.本发明提供了公式(I-A)、(I-B)和(I-C)的化合物,其药学上可接受的盐以及其制药组合物。本发明的化合物可用于抑制Myc(例如c-Myc)的活性。本发明还提供了使用本文所描述的化合物治疗Myc介导的疾病(例如癌症和其他增殖性疾病)的方法。本发明还提供了用于鉴定Myc抑制剂的检测方法。
-
Compositions and methods for treating neoplasia, inflammatory disease and other disorders申请人:Dana-Farber Cancer Institute, Inc.公开号:US10407441B2公开(公告)日:2019-09-10The invention features compositions and methods for treating or preventing a neoplasia. More specifically, the invention provides compositions and methods for disrupting the interaction of a BET family polypeptide comprising a bromodomain with chromatin (e.g., disrupting a bromodomain interaction with an acetyl-lysine modification present on a histone N-terminal tail).
表征谱图
-
氢谱1HNMR
-
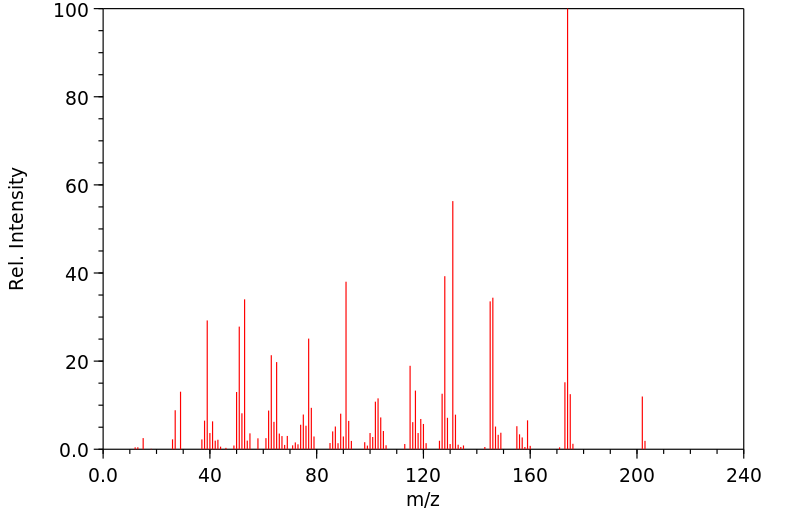
质谱MS
-
碳谱13CNMR
-
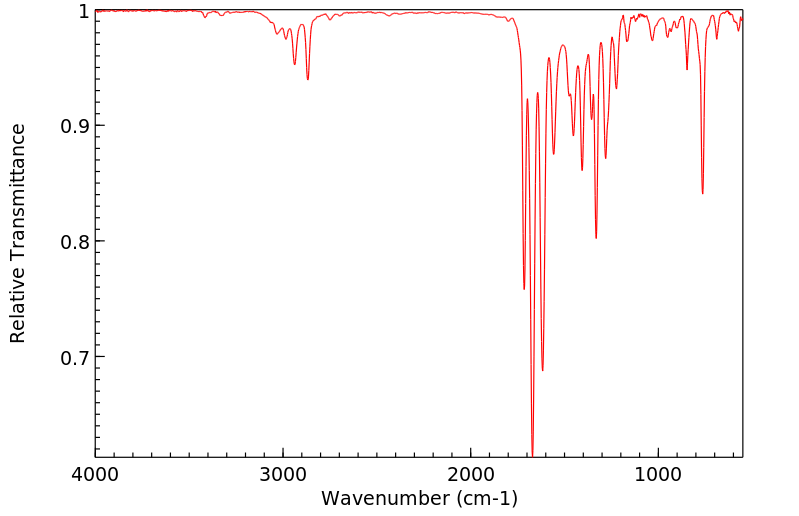
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-8-氟苯并二氢吡喃-4-胺
(2S)-6-氟-3,4-二氢-4-氧代-2H-1-苯并吡喃-2-甲酸
(2R)-6-氟-3,4-二氢-4-氧代-2H-1-苯并吡喃-2-甲酸
龙胆根素
龙胆SHAN酮
齿阿米素
黑色素-1
黄天精
麥角黃酮酸
鲍迪木醌
高邻苯二甲酸酐
高芒果苷
高氯酸罗丹明640
马佐卡林
香豆素-340
香豆素 339
食用色素红色105号
颜料红90:1铝色淀[CI45380:3]
颜料红172铝色淀[CI45430:1]
雏菊叶龙胆酮
降阿赛里奥; 1,3,6,7-四羟基氧杂蒽酮
阿米醇
阿米凯林
阿米凯林
阿扎那托
阿巴哌酮
阿尼地坦
阿尼地坦
阿匹氯铵
锌离子荧光探针-4
锆(2+)二[2-(2,4,5,7-四溴-3,6-二羟基氧杂蒽-9-基)-3,4,5,6-四氯苯甲酸酯]
铁力木呫吨酮-B
铁力木吨酮A
钠6'-羟基-5-[2-[4-(2-甲基-3-氧代-7H-咪唑并[1,2-d]吡嗪-6-基)苯氧基]乙基硫代氨基甲酰氨基]-3-氧代螺[2-苯并呋喃-1,9'-氧杂蒽]-3'-醇
钠4-{[6'-(二乙基氨基)-3'-羟基-3-氧代-3H-螺[2-苯并呋喃-1,9'-氧杂蒽]-2'-基]偶氮}-3-羟基-1-萘磺酸酯
钠2-(2,7-二氯-9H-氧杂蒽-9-基)苯甲酸酯
钙黄绿素乙酰甲酯
钙荧光探针Fluo-8,AM
钙荧光探针Fluo-4,AM
钙离子荧光探针
钙柑子
采木(HAEMATOXYLONCAMPECHIANUM)木质提取物
酸性媒介桃红3BM
邻苯三酚红
远志山酮III
转移核糖核酸(面包酵母)
赤藓红B异硫氰酸酯异构体II
赤藓红
诺大麻
西伯尔链接剂