pinacoline oxime | 10341-64-7
中文名称
——
中文别名
——
英文名称
pinacoline oxime
英文别名
pinacolone oxime;(NE)-N-(3,3-dimethylbutan-2-ylidene)hydroxylamine
CAS
10341-64-7
化学式
C6H13NO
mdl
——
分子量
115.175
InChiKey
UNSDDJQNHCSVSW-FNORWQNLSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:76 °C
-
沸点:172.2±9.0 °C(Predicted)
-
密度:0.86±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:8
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:32.6
-
氢给体数:1
-
氢受体数:2
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (E)-3,3-Dimethylbutan-2-one O-methyloxime 59170-03-5 C7H15NO 129.202
反应信息
-
作为反应物:描述:参考文献:名称:通过将三丁基锡自由基加到N-酰基丙啶上来实现均相氮丙啶的开环(环丙基羰基-高烯丙基重排的氮杂变体)。影响区域选择性的因素摘要:AIBN在回流的苯中引发N-酰基丙啶1与Bu 3 SnH的反应,提供了还原性开环的产物5和8。由于添加了Bu 3 Sn的位阻,产率(大多数情况下是定量的)急剧下降。到1的酰基氧。他们在一定程度上取决于当氮丙啶均质分解提供伯自由基3或6时捕获氢的实验条件。的(可能是可逆的)环均裂区域选择性可以的产生自由基(稳定性的观点出发可以理解3,6),立体声电子控制(例如1i与1h相比)和边界轨道相互作用(1j)。从1j形成一级自由基的解释中,键长可能存在差异,但从N-甲苯磺酰基-2-甲基-氮丙啶11的X射线结构分析中未发现支持。异构体产物仅获得两次(1i,1j),比率5j:8j取决于Bu 3 SnH的浓度和氢同位素。没有发现比例为5:14的这种依赖关系(环化和无环化时减少3)由N-肉桂酰基氮丙啶11得到的吡咯烷酮。该比率(1:9为11和1:3为1N)必须反映在EZ异构体3。所观察到的由2形成E-3的偏DOI:10.1016/s0040-4020(01)81081-4
-
作为产物:描述:参考文献:名称:用于制备由肟的 O-H 插入芳基重氮乙酸酯衍生的肟醚的可见光介导策略摘要:报道了两种涉及肟和芳基重氮乙酸酯的可见光介导的 O-H 插入方案,根据所用溶剂的不同,产生不同的产物。在 DCM 中,发生直接 O-H 插入。在 THF 中,该溶剂的开环形式额外结合到产品结构中。这些无金属方案温和且耐空气和水分。已开发出制备杀螨剂作为合成应用的一个例子。DOI:10.1021/acs.joc.1c02411
文献信息
-
Facile One-Pot Syntheses of Amidines and Enamines from Oximes via Beckmann Rearrangement Using Trifluoromethanesulfonic Anhydride作者:Tomofumi Takuwa、Tomofumi Minowa、Jim Yoshitaka Onishi、Teruaki MukaiyamaDOI:10.1246/bcsj.77.1717日期:2004.9Iminocarbocation intermediates were in situ-generated by treating various oximes with trifluoromethanesulfonic anhydride (Tf2O) in the presence of triethylamine in toluene and nucleophilic trapping with amines or sodium enolates under mild conditions afforded the corresponding amidines and enamines. Some of the thus-obtained enamines were converted to 2-substituted 4-oxo-3-quinolinecarboxylic acid derivatives by subsequent intramolecular Friedel–Crafts acylation.
-
NOVEL COMPOUNDS AS CANNABINOID RECEPTOR LIGANDS申请人:Carroll William A.公开号:US20100069348A1公开(公告)日:2010-03-18Disclosed herein are cannabinoid receptor ligands of formula (I) wherein A 1 , A 5 , R x , X 4 , and z are as defined in the specification. Compositions comprising such compounds, and methods for treating conditions and disorders using such compounds and compositions are also disclosed.
-
Beckmann Rearrangement of Oximes under Very Mild Conditions作者:Lidia De Luca、Giampaolo Giacomelli、Andrea PorchedduDOI:10.1021/jo025960d日期:2002.8.1A variety of ketoximes, easily prepared from the corresponding ketones, undergo the Beckmann rearrangement upon treatment with 2,4,6-trichloro[1,3,5]triazine in N,N-dimethylformamide at room temperature in excellent yields. This procedure can be applied to aldoximes for obtaining the corresponding nitriles.
-
IMPROVED METHOD FOR PRODUCING SPECIFIC OXIMES AND OXIMETHERS申请人:BAYER CROPSCIENCE Aktiengesellschaft公开号:US20160107986A1公开(公告)日:2016-04-21Method for preparing certain oximes and oxime O-methyl ethers by reacting poorly water-soluble carbonyl compounds with salts of hydroxylamine or hydroxylamine O-methyl ether or the free base of hydroxylamine in the presence of certain phosphoric esters or salts thereof of the formula (I) wherein R 1 , R 2 and X are defined as specified in the description.
-
From ( E )- and ( Z )-ketoximes to N -sulfenylimines, ketimines or ketones at will. Application to erythromycin derivatives作者:Jorge Esteban、Anna M. Costa、Fèlix Urpı́、Jaume VilarrasaDOI:10.1016/j.tetlet.2004.06.002日期:2004.7disulfide (PhSSPh) have been compared to gain insight into the mechanisms involved and their potential applications. N-Sulfenylimine isomers and ketimines have been spectroscopically characterised. Both the E and Z isomers of erythromycin A oxime, when treated with Bu3P and PhSSPh (1:4:8 ratio), give the same N-phenylsulfenyl ketimine (of configuration E) as the major compound, whereas with Bu3P or
表征谱图
-
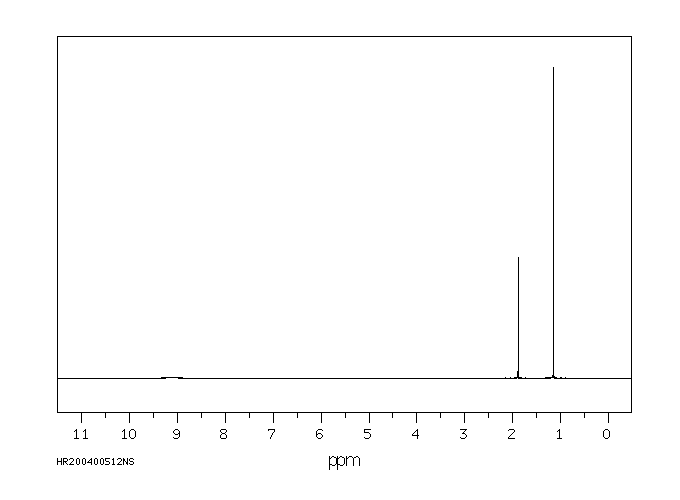
氢谱1HNMR
-
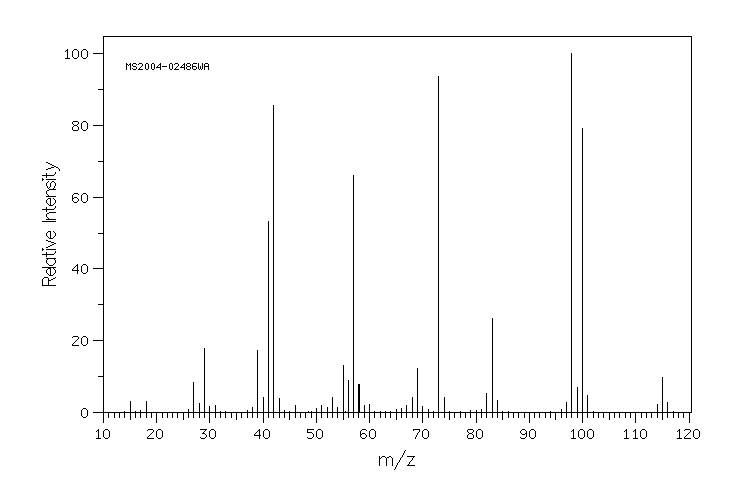
质谱MS
-
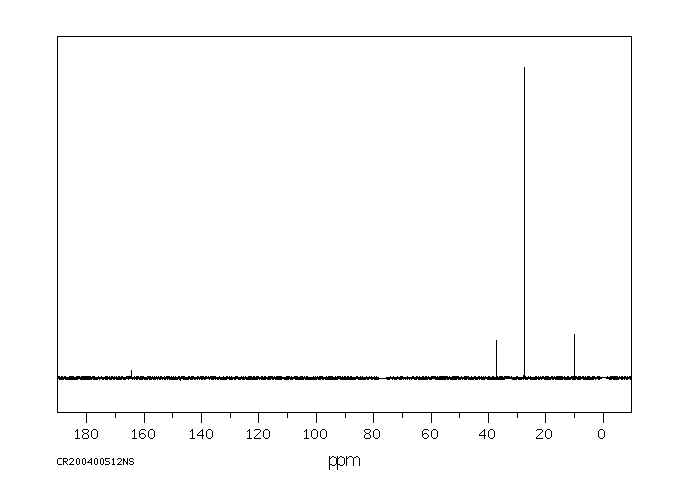
碳谱13CNMR
-
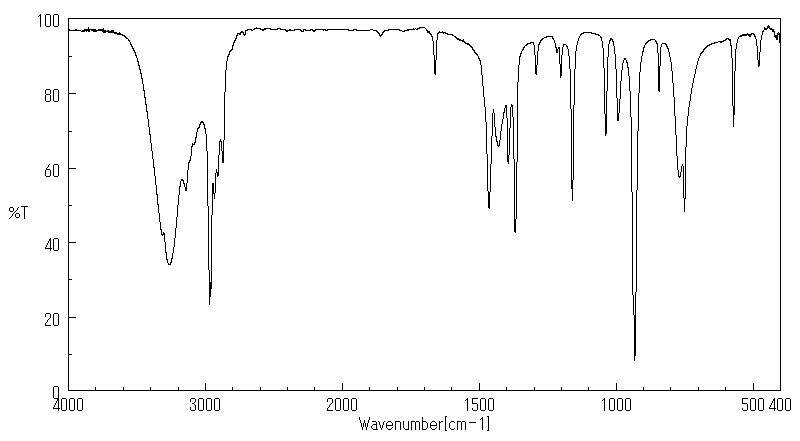
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷