乙炔二羰酰胺 | 543-21-5
中文名称
乙炔二羰酰胺
中文别名
叶枯炔;乙炔二羧酰胺
英文名称
Cellocidin
英文别名
acetylenedicarboxamide;Acetylen-dicarbonsaeurediamid;acetylenedicarboxylic acid diamide;but-2-ynediamide
CAS
543-21-5
化学式
C4H4N2O2
mdl
MFCD00017146
分子量
112.088
InChiKey
JBTGHKUTYAMZEZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:179 °C
-
沸点:209.98°C (rough estimate)
-
密度:1.4421 (rough estimate)
-
溶解度:DMF:10mg/mL; DMSO:20mg/mL; DMSO:PBS (pH 7.2) (1:7):0.12 mg/mL
-
稳定性/保质期:
在常温常压下保持稳定,应避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):-1.2
-
重原子数:8
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:86.2
-
氢给体数:2
-
氢受体数:2
安全信息
-
危险品标志:T
-
安全说明:S28A,S36/37,S45
-
危险类别码:R25,R21
-
海关编码:2924199090
-
储存条件:密封保存,应储存在阴凉干燥的仓库中。
SDS
| Name: | Acetylenedicarboxamide (pract) Material Safety Data Sheet |
| Synonym: | 2-Butynediamide; Cellocidin |
| CAS: | 543-21-5 |
Synonym:2-Butynediamide; Cellocidin
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 543-21-5 | Acetylenedicarboxamide, pract. | >98 % | unlisted |
Risk Phrases: 21 25
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful in contact with skin. Toxic if swallowed.Toxic.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. Harmful if absorbed through the skin.
Ingestion:
May be fatal if swallowed. Harmful if swallowed. Causes gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
Causes respiratory tract irritation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately. SPEED IS ESSENTIAL.
A DOCTOR MUST BE NOTIFIED AT ONCE.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Wash hands before eating. Remove contaminated clothing and wash before reuse. Use only in a well-ventilated area. Do not get on skin or in eyes. Do not ingest or inhale.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 543-21-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves and clothing to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 179 deg C
Autoignition Temperature: Not available.
Flash Point: 216 deg C ( 420.80 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: 216 deg C
Solubility in water: slightly soluble
Specific Gravity/Density:
Molecular Formula: C4H4N2O2
Molecular Weight: 112.09
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 543-21-5: AO9900000 LD50/LC50:
CAS# 543-21-5: Oral, mouse: LD50 = 89200 ug/kg.
Carcinogenicity:
Acetylenedicarboxamide, pract. - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: T
Risk Phrases:
R 21 Harmful in contact with skin.
R 25 Toxic if swallowed.
Safety Phrases:
S 28A After contact with skin, wash immediately with
plenty of water.
S 36/37 Wear suitable protective clothing and
gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 543-21-5: No information available.
Canada
CAS# 543-21-5 is listed on Canada's NDSL List.
CAS# 543-21-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 543-21-5 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
类别:农药
毒性分级:高毒
急性毒性(口服):小鼠 LD50 为 89.2 毫克/公斤
可燃性危险特性:燃烧时会产生有毒氮氧化物气体
储运特性:需存放在通风、低温和干燥的库房中,并与食品原料分开储存运输
灭火剂:干粉、泡沫或砂土
反应信息
-
作为反应物:参考文献:名称:一种新的和潜在的益生元α-胞苷衍生物。摘要:由核糖氨基恶唑啉和二氰基乙炔的益生元反应合成了一种新的α-胞苷衍生物。通过 X 射线衍射证实了产物的四环结构,然后找到了适合大规模合成的替代 6 步合成途径。DOI:10.1039/c7cc00693d
-
作为产物:描述:参考文献:名称:Kharasch; Jensen; Urry, Journal of Organic Chemistry, 1945, vol. 10, p. 392摘要:DOI:
文献信息
-
Organic heterocyclothiazenes. Part 5. Cycloaddition reactions of tetrasulphur tetranitride with highly electron deficient alkynes作者:Peter J. Dunn、Charles W. ReesDOI:10.1039/p19870001579日期:——4-trifluorobutynonitrile (4) similarly give the trithiadiazepines (10) and (12), though more slowly and in slightly lower yields. Structures (8) and (10) correct literature assignments. The analogous reactions of ethyl 3-formylpropynoate (6), hex-3-yne-2,5-dione (7), and butynedial, like those of acetylenedicarboxylate esters, give complex mixtures from which trithiadiazepines, trithiatriazepines, and thiadiazoles与先前的复杂S 4 N 4-炔反应相反,丁腈和S 4 N 4的处理可得到1,3,5,2,4-三噻二氮杂-6,7-二碳腈(8)和1, 2,5-噻二唑-3,4-二甲腈(9)非常干净,收率很高。六氟丁-2-炔和4,4,4-三氟丁腈(4)类似地产生三噻二氮杂(10)和(12),尽管速度较慢且收率略低。结构(8)和(10)纠正文献分配。3-甲酰基丙酸乙酯(6),己-3-炔-2,5-二酮(6)的类似反应7),丁炔和乙炔二羧酸酯一样,得到复杂的混合物,从中可以不同地分离出三噻二氮杂,三噻三氮杂和噻二唑。建议对这些结果进行机械合理化。由S 4 N 4和1,1,4,4-四乙氧基丁炔(20)以高收率制备1,2,5-噻二唑-3,4-二甲醛(18b),然后进行水解。该二醛和其他3,4-二氧代-1,2,5-噻二唑首次用于制备1,2,5-噻二唑并[3,4- d ]哒嗪及其衍生物(22)。改进的3-甲酰基丙酸乙酯(6)和十六烷基-3-炔-2
-
Pesticides申请人:Rhone-Poulenc, Inc.公开号:US06346522B1公开(公告)日:2002-02-12Compounds of formula (I) or pesticidally acceptable salts thereof, compositions containing them and methods of use.式(I)的化合物或其农药可接受的盐,含有它们的组合物以及使用方法。
-
Compound and method of producing organic semiconductor device申请人:Canon Kabushiki Kaisha公开号:US07928221B2公开(公告)日:2011-04-19A method of producing an organic semiconductor device is provided in which a layer composed of an organic semiconductor having excellent crystallinity and orientation in a low-temperature region can be formed, and the device can be produced in the air. The method includes forming a layer composed of an organic semiconductor precursor on a base body and irradiating the organic semiconductor precursor with light, wherein the organic semiconductor precursor is a porphyrin compound or an azaporphyrin compound having in its molecule at least one of the structure represented by the following general formula (1) or (2):
-
Electron spin resonance investigation of sulphur-33 and nitrogen-15 substituted dithiazol-2-yl and dithiazolidin-2-yl free radicals作者:Shirley A. Fairhurst、Roger S. Pilkington、Leslie H. SutcliffeDOI:10.1039/f19837900925日期:——Thermal cyclo-addition reactions of tetrasulphur dinitride with certain alkynes generate a new series of stable free radicals possessing the 1,3,2-dithiazolyl ring. The radicals have simple e.s.r. spectra and are potentially useful spin probes or labels. The sharpness of the spectral lines has enabled us to observe natural abundance lines from nitrogen-15, sulphur-33 and carbon-13. The presence of
-
NIR chromophores from small acetylenic building blocks: a Diels–Alder approach to octaalkynylphthalocyanines作者:Rüdiger Faust、Frieder MitzelDOI:10.1039/b006114j日期:——Two new routes to cross-conjugated 3,4-dimethylenehexa-1,5-diynes, both starting from dialkynyl 1,2-diones, have been devised. Whereas triisopropylsilyl-protected diketones could be diolefinated in a bis-Wittig reaction with methylenetriphenylphosphorane, their aryl-terminated congeners had to be subjected to the conditions of the Peterson olefination (trimethylsilylmethylmagnesium chloride and subsequent dehydration of the resulting diol with thionyl chloride). The reactivity of the diethynylbutadienes towards standard dienophiles was found to be low and alternative thermal reactions compete with [4Â +Â 2] cycloadditions. However, dicyanoacetylene was shown to be an effective cycloaddition partner leading, after aromatisation, to the corresponding dicyanodiethynylbenzenes. These were cyclotetramerised with magnesium butanolate in butanol to furnish octaalkynylphthalocyanines, thereby completing a concise, three-step synthesis of these NIR chromophores of relevance to photodynamic forms of therapy.研究人员设计了两条新的路线,从 1,2 二炔基二酮出发,制备交叉共轭的 3,4-二亚甲基六-1,5-二炔。三异丙基硅烷保护的二酮类化合物可以在与亚甲基三苯基膦的双维蒂希反应中进行二烯化反应,而其芳基封端的同系物则必须在彼得森烯化反应的条件下进行(三甲基硅甲基氯化镁,然后用亚硫酰氯对生成的二元醇进行脱水)。研究发现,二炔丁二烯与标准亲二烯的反应活性较低,替代热反应与[4Â +Â 2]环加成反应存在竞争。不过,二氰基乙炔被证明是一种有效的环加成伙伴,经过芳香化后可生成相应的二氰基二炔基苯。这些化合物在丁醇中与丁酸镁发生环四聚反应,生成八烷基炔基酞菁,从而完成了与光动力疗法相关的近红外发色团的简明三步合成。
表征谱图
-
氢谱1HNMR
-
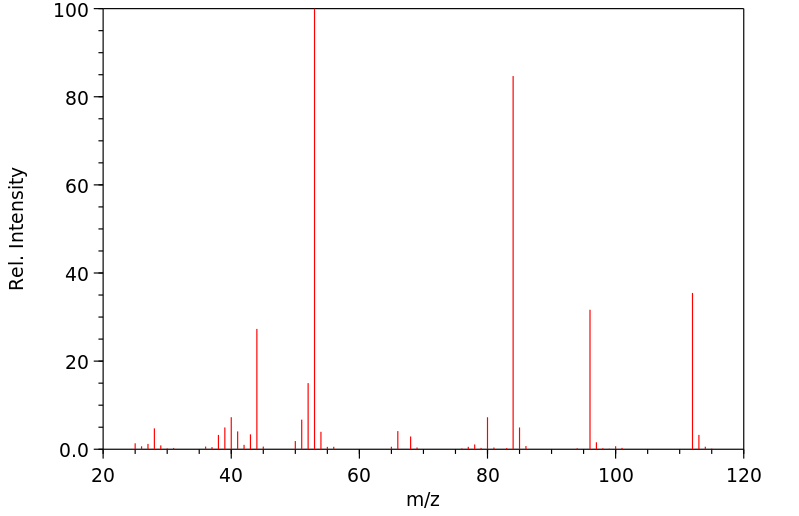
质谱MS
-
碳谱13CNMR
-
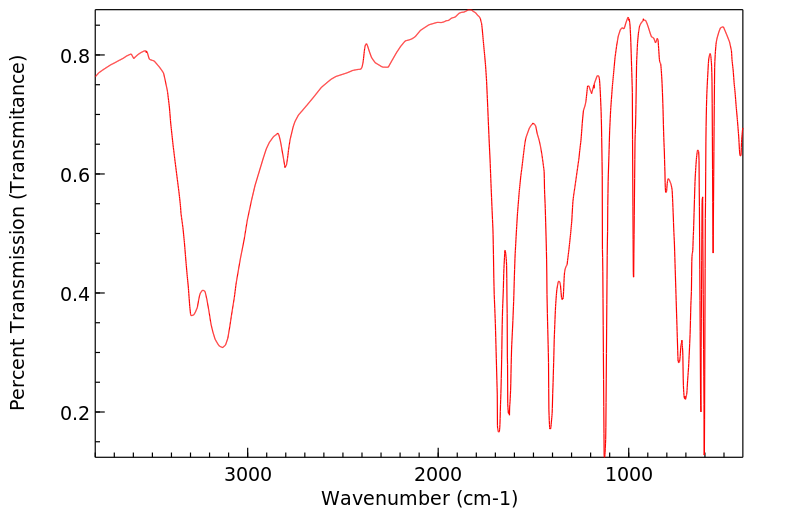
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
辛二亚氨酸二甲酯二盐酸盐
辛二亚氨酸二甲酯
脲鎓溴化物
羟甲基脲
羟基脲
缩二脲
缩三脲
碳亚胺酸二丙酯
硬脂酰胺
癸醯胺
甲酰胺-d3
甲酰胺
甲基氰基脒醚
甲基双环[2.2.1]庚-5-烯-2-甲亚氨酸酯
甲基乙酰亚胺酯盐酸盐
甲基N-氰基-N'-甲基氨基亚胺酸酯
甲基N-异丙基-N-甲基氨基亚胺酸酯
甲基3-氯代丙酸乙酯盐酸盐
甲基2-氯乙亚氨酸酯盐酸盐
甲基2,2-二乙氧基乙亚氨酸酯
甲基(3-甲基-1-硫基-3-丁烯-2-基)氨基甲酸酯
甲亚胺异丙酯 盐酸盐
甲亚胺乙酯盐酸盐
甘氨酰胺
环戊烷甲亚氨酸乙酯
环丙酰胺
环丙烷甲亚胺酸乙酯
溴米索伐
涕灭威
氰基甲酯
氰基亚氨代甲酸甲酯
氰基乙酯
氨基甲酸乙酯
氨基丙酮缩氨基脲盐酸盐
氟乙酰胺
戊亚氨酸甲酯
异丙基氨基亚胺酸酯盐酸盐(1:1)
庚二亚氨酸二甲酯二盐酸盐
叔丁基三氯乙酰亚胺酯
十六酰胺乙醇
亚油酰胺
亚氨酰乙酸甲酯
亚氨戊酸甲酯盐酸盐
亚氨基碳酸二甲酯
二硫代二丙亚氨酸二甲酯
二乙氧基甲亚胺
二乙基丙烷二亚氨酸酯二盐酸盐
乙酰胺
乙炔二羰酰胺
乙氧亚氨基乙酸乙酯