1-(苯氧甲基)萘 | 6245-96-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.7
-
重原子数:18
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.058
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-萘甲醇 1-naphthalene methanol 4780-79-4 C11H10O 158.2
反应信息
-
作为反应物:参考文献:名称:自由基阴离子裂解中的电子分配。2. 萘甲基苯基醚与萘基苄基醚摘要:Les ethers du titre subissent une scission de la liaison H 2 CO lorsqu'ils sont traites par les radicaux anioniques de l'anthracene ou du fluoranthrene。Dans des conditions 可比 les naphtylmethyl phenyl ethers reagisent plus de 10 4 fois plus vite que lesnaphtylbenzyl ethers。解释DOI:10.1021/ja00270a022
-
作为产物:描述:参考文献:名称:苄基sulf盐的合成和反应性:苯酚和苯硫酚在近中性条件下的苄基化摘要:从三元体系ArCH 2 OH:H 2 SO 4:Me 2 S或四氢噻吩合成了一系列苄基二甲基ulf盐和相关的硫酸氢盐。所述盐通常是稳定的结晶固体,但是对于9-(蒽甲基)二甲基ulf硫酸氢盐观察到异常高的反应性。在接近中性的两相体系中,已选择的sulf盐分别用于苯酚和硫酚的O-和S-苄基化。还描述了在碱性条件下肟和苯并咪唑的苄基化。DOI:10.1016/s0040-4020(01)00137-5
文献信息
-
Tetrabutyl ammonium bromide-mediated benzylation of phenols in water under mild condition作者:Hailei Wang、Yuping Ma、Heng Tian、Ajuan Yu、Junbiao Chang、Yangjie WuDOI:10.1016/j.tet.2014.01.004日期:2014.4Benzylation of phenol was successfully achieved in water under room temperature mediated by tetrabutylammonium bromide (TBAB) for only 2 h affording the corresponding benzyl phenyl ether with good to excellent yields. This protocol is very efficient, simple, avoiding catalysts, easy to work-up after reaction, and especially ‘green’.
-
Metal-Free C–O Bond Functionalization: Catalytic Intramolecular and Intermolecular Benzylation of Arenes作者:Luis Bering、Kirujan Jeyakumar、Andrey P. AntonchickDOI:10.1021/acs.orglett.8b01495日期:2018.7.6conditions enabled a catalytic and metal-free Friedel–Crafts alkylation reaction with benzylic alcohols, producing water as the stoichiometric byproduct. A comprehensive scope (>50 examples) for both approaches and application in drug synthesis were demonstrated. Mechanistic studies suggest a Lewis acid-based mechanism for the metal-free Friedel–Crafts reaction.
-
From insertion to multicomponent coupling: temperature dependent reactions of arynes with aliphatic alcohols作者:Manikandan Thangaraj、Sachin Suresh Bhojgude、Manoj V. Mane、Akkattu T. BijuDOI:10.1039/c5cc08307a日期:——
The temperature dependent switchable reactivity of arynes with aliphatic alcohols in THF has been demonstrated.
芳炔与脂肪族醇在THF中的温度依赖性可切换反应性已被证明。 -
Palladium-Catalyzed Substitution and Cross-Coupling of Benzylic Fluorides作者:George Blessley、Patrick Holden、Matthew Walker、John M. Brown、Véronique GouverneurDOI:10.1021/ol300977f日期:2012.6.1Benzylic fluorides are suitable substrates for Pd(0)-catalyzed Tsuji–Trost substitution using carbon, nitrogen, oxygen, and sulfur nucleophiles and for cross-coupling with phenylboronic acid. For the bifunctional substrate 4-chlorobenzyl fluoride, fine-tuning of the reaction conditions allows for the regioselective displacement of either the chlorine or fluorine substituent. The leaving group ability
-
The photochemistry of 1-naphthylmethyl carbonates and carbamates作者:T. Parman、J.A. Pincock、P.J. WedgeDOI:10.1139/v94-159日期:1994.5.1
The photochemistry in methanol of 1-naphthylmethyl phenyl carbonate (3) and 1-naphthylmethyl benzyl carbonate (4) has been studied. Products resulting from both the 1-naphthylmethyl cation and the 1-naphthylmethyl radical are obtained for 3, but only from the cation for 4. Similar results were obtained for the corresponding 1-naphthylmethyl derivatives 5 and 6 of N-phenyl and N-benzyl carbamic acids. The product yields for all four compounds can be explained by a mechanism of initial homolytic cleavage of the 1-naphthylmethyl carbon–oxygen bond from the excited singlet state. The radical pair generated then partitions between the two pathways: electron transfer to form the ion pair or decarboxylation. For PhO-CO-O• and PhNH-CO-O•, decarboxylation is rapid and competitive with electron transfer. For PhCH2O-CO-O• and PhCH2NH-CO-O•, decarboxylation is slower, electron transfer dominates, and only products from the ion pair are obtained.
表征谱图
-
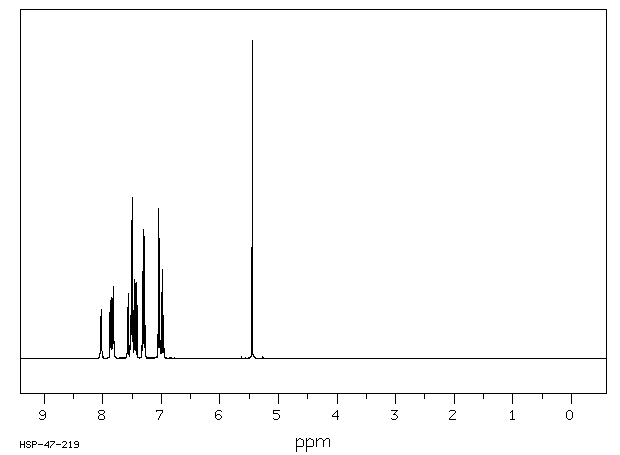
氢谱1HNMR
-
质谱MS
-
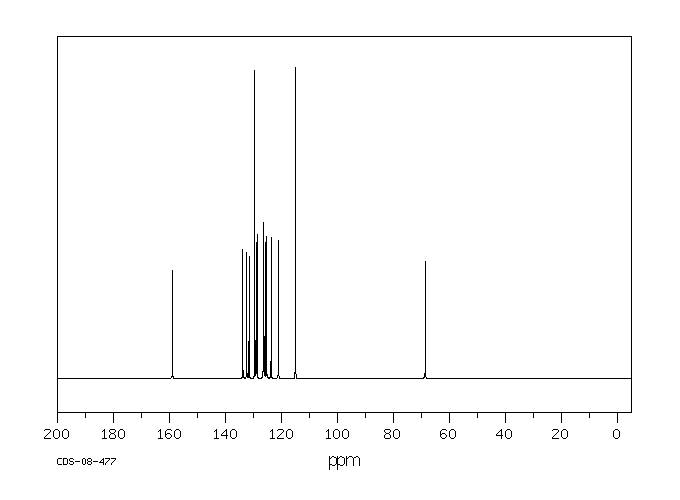
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息