癸基三氯硅烷 | 13829-21-5
中文名称
癸基三氯硅烷
中文别名
正癸基三氯硅烷;三氯癸基硅烷
英文名称
n-decyltrichlorosilane
英文别名
decyltrichlorosilane;Trichlor-decyl-silan;trichloro(decyl)silane
CAS
13829-21-5
化学式
C10H21Cl3Si
mdl
MFCD00013600
分子量
275.721
InChiKey
HLWCOIUDOLYBGD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:135 °C5 mm Hg(lit.)
-
密度:1.054 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):5.52
-
重原子数:14
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
危险等级:8
-
危险品标志:C
-
安全说明:S26,S27,S36/37/39,S45
-
危险类别码:R34
-
WGK Germany:1
-
海关编码:2931900090
-
危险品运输编号:UN 2987 8/PG 2
-
包装等级:II
-
危险类别:8
-
储存条件:常温密闭,阴凉通风干燥的惰性气体环境中保存。
SDS
Decyltrichlorosilane Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Decyltrichlorosilane
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 1
Corrosive to metals
HEALTH HAZARDS
Category 1B
Skin corrosion/irritation
Serious eye damage/eye irritation Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements May be corrosive to metals
Causes severe skin burns and eye damage
Precautionary statements:
[Prevention] Keep only in original container.
Do not breathe dust/fume/gas/mist/vapours/spray.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Absorb spillage to prevent material damage.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Decyltrichlorosilane
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Decyltrichlorosilane
Percent: >94.0%(GC)
CAS Number: 13829-21-5
Synonyms: Trichlorodecylsilane
Chemical Formula: C10H21Cl3Si
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, carbon dioxide.
media:
Unsuitable extinguishing Water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (self-contained breathing apparatus). Keep
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Prevention of secondary Do not allow contact with water. Remove all sources of ignition. Fire-extinguishing
hazards: devices should be prepared in case of a fire. Use spark-proof tools and explosion-
proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Decyltrichlorosilane
Section 7. HANDLING AND STORAGE
May develop pressure. Open carefully.
Use corrosive resistant equipment.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Protect from moisture.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Moisture-sensitive
Packaging material: Comply with laws. Keep only in original container.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Impervious gloves.
Hand protection:
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Slightly pale yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
96°C/0.3kPa
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
Relative density: 1.05
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous Decomposes in contact with water and liberates toxic gases.
reactions:
Conditions to avoid: Moisture
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride, Silicon oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
Decyltrichlorosilane
Section 11. TOXICOLOGICAL INFORMATION
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 8: Corrosive.
UN-No: 2987
Proper shipping name: Chlorosilanes, corrosive, n.o.s.
II
Packing group:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Decyltrichlorosilane
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 1
Corrosive to metals
HEALTH HAZARDS
Category 1B
Skin corrosion/irritation
Serious eye damage/eye irritation Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements May be corrosive to metals
Causes severe skin burns and eye damage
Precautionary statements:
[Prevention] Keep only in original container.
Do not breathe dust/fume/gas/mist/vapours/spray.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Absorb spillage to prevent material damage.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Decyltrichlorosilane
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Decyltrichlorosilane
Percent: >94.0%(GC)
CAS Number: 13829-21-5
Synonyms: Trichlorodecylsilane
Chemical Formula: C10H21Cl3Si
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, carbon dioxide.
media:
Unsuitable extinguishing Water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (self-contained breathing apparatus). Keep
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Prevention of secondary Do not allow contact with water. Remove all sources of ignition. Fire-extinguishing
hazards: devices should be prepared in case of a fire. Use spark-proof tools and explosion-
proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Decyltrichlorosilane
Section 7. HANDLING AND STORAGE
May develop pressure. Open carefully.
Use corrosive resistant equipment.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Protect from moisture.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Moisture-sensitive
Packaging material: Comply with laws. Keep only in original container.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Impervious gloves.
Hand protection:
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Slightly pale yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
96°C/0.3kPa
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
Relative density: 1.05
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous Decomposes in contact with water and liberates toxic gases.
reactions:
Conditions to avoid: Moisture
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride, Silicon oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
Decyltrichlorosilane
Section 11. TOXICOLOGICAL INFORMATION
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 8: Corrosive.
UN-No: 2987
Proper shipping name: Chlorosilanes, corrosive, n.o.s.
II
Packing group:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
反应信息
-
作为反应物:参考文献:名称:设计的具有烷基链的低聚硅氧烷自组装成基于二氧化硅的混合介观结构摘要:已经研究了使用具有烷氧基官能团和共价连接的烷基链的明确定义的硅氧烷低聚物来制备有序二氧化硅-有机杂化物的新型自组装途径。在不使用任何结构导向剂的情况下,通过水解和缩聚获得了各种混合介观结构。具有烷基硅烷核和三个支链三甲氧基甲硅烷基的低聚物 1(Cn) 在 n = 14-18 时形成高度有序的层状相,而那些具有较短烷基链的低聚物形成圆柱形组件,略微扭曲的二维 (2D) 六边形结构( n = 6-10),以及一种新颖的二维单斜结构(n = 12)。此外,具有不同链长的 1(Cn) 的混合物产生了有序的二维六方相,这可能是由于前体更好的堆积。在煅烧以去除有机基团时,由圆柱形组件组成的杂化物被转化为具有可调孔径的有序多孔二氧化硅。1(Cn) 的水解和缩聚过程的液态 29Si NMR 分析揭示了一种独特的分子内反应,主要产生具有四硅氧烷环的低聚物,这是一类具有自组装能力和高交联性的新型两亲分子能力。DOI:10.1021/ja0541736
-
作为产物:参考文献:名称:螺旋魔法。柔性杆螺旋聚硅烯的热驱动手性开关和螺旋感应反转摘要:一种理想的光学活性螺旋发色聚合物,包含柔性棒状硅主链和对映纯烷基侧链,聚{(S)-3,7-二甲基辛基-3-甲基丁基亚硅烷},在-20°经历热驱动螺旋-螺旋转变C 在异辛烷中。通过光谱分析圆二色性 (CD) 和紫外吸收特性,可以定量表征转变特征,包括转变温度、转变宽度、右手和左手螺旋图案的数量、全局形状和螺距。这是基于棒状聚合物的独特性质,其中 CD 波段在所有温度下都与相应的 UV 波段轮廓完全匹配。而且,精细控制掺入共聚物中的额外手性亚硅烷单元的含量和手性允许在 -64 至 +79 °C 范围内自由控制转变温度。分子力学计算表明势能曲线存在显着差异...DOI:10.1021/ja9938581
文献信息
-
Processes for manufacturing organochlorosilanes and dipodal silanes and silanes made thereby申请人:Janeiro A. Benigno公开号:US20050027138A1公开(公告)日:2005-02-03Processes are provided for producing organchlorosilanes and dipodal silanes in which an organic halide or alkene or chloralkene is reacted with a hydridochlorosilane in the presence of a quarternary phosphonium salt catalyst by providing sufficient heat to effect a dehydrohalogenative coupling reaction and/or a hydrosilylation reaction and venting the reaction to control reaction pressure and to remove gaseous byproducts from the reaction. The processes are preferably continuous using a catalyst in fluid form at reaction pressures not exceeding about 600 psi. The reactions may be carried out substantially isothermally and/or isobarically, for example in a plug flow reactor or continuous stirred tank reactor. The processes may produce novel silylated compounds including 1,2-bis(trichlorosilyl)decane or 1,2-bis(trimethoxysilyl)decane.
-
(Halophenoxy)silanes. VIII作者:Roshdy M. Ismail、Hans J. KoetzschDOI:10.1016/s0022-328x(00)83165-4日期:1967.12A new simple process for the preparation of silane esters of halogenated phenols is described. The reaction of halogenated phenols and chlorosilanes is catalysed by certain types of amines to produce nearly quantitative yields of halogenated phenoxysilanes.
-
硅酮密封胶交联剂及其制备方法和应用
-
A General and Selective Synthesis of Methylmonochlorosilanes from Di-, Tri-, and Tetrachlorosilanes作者:Yuki Naganawa、Kei Sakamoto、Yumiko NakajimaDOI:10.1021/acs.orglett.0c04175日期:2021.1.15Direct catalytic transformation of chlorosilanes into organosilicon compounds remains challenging due to difficulty in cleaving the strong Si–Cl bond(s). We herein report the palladium-catalyzed cross-coupling reaction of chlorosilanes with organoaluminum reagents. A combination of [Pd(C3H5)Cl]2 and DavePhos ligand catalyzed the selective methylation of various dichlorosilanes 1, trichlorosilanes 5
-
Chiroptical generation and inversion during the mirror-symmetry-breaking aggregation of dialkylpolysilanes due to limonene chirality作者:Yoko Nakano、Fumiko Ichiyanagi、Masanobu Naito、Yonggang Yang、Michiya FujikiDOI:10.1039/c2cc17845a日期:——We observed the emergence and inversion of chiroptical handedness in three chiroptically silent dialkylpolysilanes during aggregation in limonene–methanol–THF tersolvents.
表征谱图
-
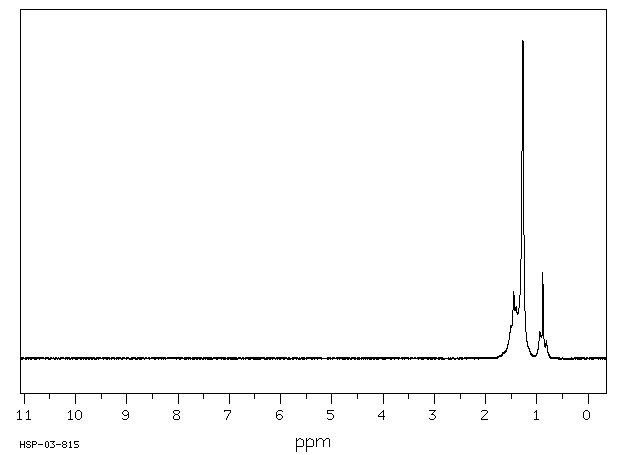
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
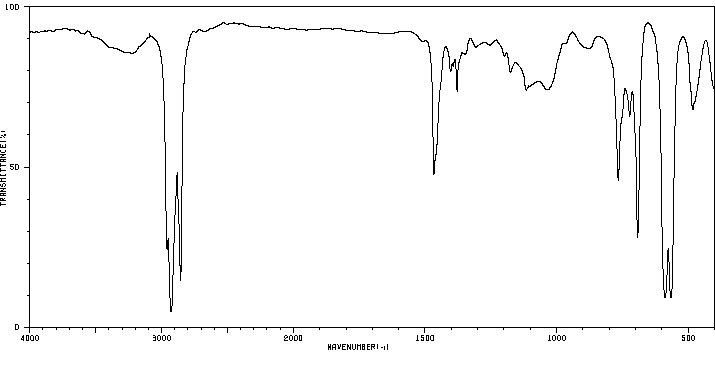
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-溴乙氧基)-特丁基二甲基硅烷
鲸蜡基聚二甲基硅氧烷
骨化醇杂质DCP
马沙骨化醇中间体
马来酸双(三甲硅烷)酯
顺式-二氯二(二甲基硒醚)铂(II)
顺-N-(1-(2-乙氧基乙基)-3-甲基-4-哌啶基)-N-苯基苯酰胺
降钙素杂质13
降冰片烯基乙基三甲氧基硅烷
降冰片烯基乙基-POSS
间-氨基苯基三甲氧基硅烷
镓,二(1,1-二甲基乙基)甲基-
镁,氯[[二甲基(1-甲基乙氧基)甲硅烷基]甲基]-
锑,二溴三丁基-
铷,[三(三甲基甲硅烷基)甲基]-
铂(0)-1,3-二乙烯-1,1,3,3-四甲基二硅氧烷
钾(4-{[二甲基(2-甲基-2-丙基)硅烷基]氧基}-1-丁炔-1-基)(三氟)硼酸酯(1-)
金刚烷基乙基三氯硅烷
酰氧基丙基双封头
达格列净杂质
辛醛,8-[[(1,1-二甲基乙基)二甲基甲硅烷基]氧代]-
辛甲基-1,4-二氧杂-2,3,5,6-四硅杂环己烷
辛基铵甲烷砷酸盐
辛基衍生化硅胶(C8)ZORBAX?LP100/40C8
辛基硅三醇
辛基甲基二乙氧基硅烷
辛基三甲氧基硅烷
辛基三氯硅烷
辛基(三苯基)硅烷
辛乙基三硅氧烷
路易氏剂-3
路易氏剂-2
路易士剂
试剂Cyanomethyl[3-(trimethoxysilyl)propyl]trithiocarbonate
试剂3-[Tris(trimethylsiloxy)silyl]propylvinylcarbamate
试剂3-(Trimethoxysilyl)propylvinylcarbamate
试剂2-(Trimethylsilyl)cyclopent-2-en-1-one
试剂11-Azidoundecyltriethoxysilane
西甲硅油杂质14
衣康酸二(三甲基硅基)酯
苯胺,4-[2-(三乙氧基甲硅烷基)乙基]-
苯磺酸,羟基-,盐,单钠聚合甲醛,1,3,5-三嗪-2,4,6-三胺和脲
苯甲醇,a-[(三苯代甲硅烷基)甲基]-
苯并磷杂硅杂英,5,10-二氢-10,10-二甲基-5-苯基-
苯基二甲基氯硅烷
苯基二甲基乙氧基硅
苯基二甲基(2'-甲氧基乙氧基)硅烷
苯基乙酰氧基三甲基硅烷
苯基三辛基硅烷
苯基三甲氧基硅烷