trans-4-(4'-Fluorophenyl)-3-hydroxymethyl-N-methyl piperidine | 318279-38-8
中文名称
——
中文别名
——
英文名称
trans-4-(4'-Fluorophenyl)-3-hydroxymethyl-N-methyl piperidine
英文别名
(4-(4-fluorophenyl)-1-methylpiperidin-3-yl)methanol;trans 4-(4'-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine;4-(4'-fluorophenyl)-3-hydroxymethyl-1-methyl-piperidine;cis-4-p-Fluorophenyl-3-hydroxymethyl-1-methylpiperidine;trans-(4R,3S)-[4-(4-fluoro-phenyl)-1-methyl-piperidin-3-yl]-methanol;(+/-)-trans-4-(4'-fluorophenyl)-3-hydroxymethyl-N-methylpiperidine;[4-(4-fluorophenyl)-1-methylpiperidin-3-yl]methanol
CAS
318279-38-8
化学式
C13H18FNO
mdl
——
分子量
223.29
InChiKey
CXRHUYYZISIIMT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:300.3±42.0 °C(Predicted)
-
密度:1.092±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:16
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.54
-
拓扑面积:23.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险类别码:R22,R41,R51/53
-
危险品运输编号:UN3077
-
海关编码:2933399090
-
包装等级:III
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 [4-(4-氟苯基)-3-哌啶基]甲醇 (3RS,4SR)-[4-(4-fluoro-phenyl)-piperidin-3-yl]-methanol 216690-19-6 C12H16FNO 209.264 —— 4-(4-fluorophenyl)-1-methyl-piperidine-3-carboxylic acid methyl ester 315197-82-1 C14H18FNO2 251.301 4-(4-氟苯基)-1-甲基-2,6-二氧代哌啶-3-羧酸乙酯 ethyl 4-(4-fluorophenyl)-1-methyl-2,6-dioxopiperidine-3-carboxylate 202534-94-9 C15H16FNO4 293.295 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-(4-氟苯)-1-甲基-3-[(4-甲苯磺酰氧基)甲基]哌啶 ((3S,4R)-4-(4-fluorophenyl)-1-methylpiperidin-3-yl)methyl 4-methylbenzenesulfonate 317323-77-6 C20H24FNO3S 377.48
反应信息
-
作为反应物:参考文献:名称:Novel process摘要:一种制备式(1)化合物的方法:其中R1是烷基,芳基烷基,烯丙基,烷氧羰基,芳基烷氧羰基,酰基或炔基;R2是取代苯基,特别是3,4-亚甲二氧基苯基;X是氢或易于去除的基团,例如氯,溴或碘。公开号:US20030187269A1
-
作为产物:描述:4-(4-氟苯基)-1-甲基-2,6-二氧代哌啶-3-羧酸乙酯 在 sodium hydroxide 作用下, 以 四氢呋喃 、 水 为溶剂, 以65%的产率得到trans-4-(4'-Fluorophenyl)-3-hydroxymethyl-N-methyl piperidine参考文献:名称:Process for preparing aryl-piperidine carbinols and novel intermediates摘要:公开了一种制备化合物的过程,其化学式为(I):其中Ar是芳基或取代芳基,R.sup.3是氢、烷基或芳基烷基,该过程包括还原化合物的过程的化学式(II):其中Ar和R.sup.3如与式(I)相关定义,R.sup.4是烷基。化合物的化学式(I)可用作化学中间体。公开号:US04902801A1
文献信息
-
[EN] ENANTIOSPECIFIC PROCESS FOR THE PREPARATION OF PAROXETINE INTERMEDIATE<br/>[FR] PROCEDE ENANTIOSPECIFIQUE PERMETTANT DE PREPARER UN INTERMEDIAIRE DE PAROXETINE申请人:NATCO PHARMA LTD公开号:WO2005063707A1公开(公告)日:2005-07-14A novel, improved, and enantiospecific process for the preparation of (-)-trans-4-(4-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine of formula-(I), an advanced intermediate in the manufacture of antidepressant drug paroxetine is disclosed in the present invention. Compound of formula-(XXII) is prepared by resolution of compound of formula-(XX) using a chiral acid followed by hydrogenation of the resolved amine. Michael addition of the compound of formula-(XXII) onto acrylate esters gave the compounds of formula-(XXIII). Conversion of the hydroxy group present in compound of formula-(XXIII) into a leaving group followed by treatment with a strong base gave the enantiospecific intramolecularly cyclized piperidine derivative of formula-(XXV). Reduction of the ester group present in compound of formula-(XXV) with a metal hydride reducing agent gave the compound of formula-I with more than 97% chiral purity. Further purification of compound of formula-I to >99.5% is achieved by one recrystallization from a number of solvents. Present process is easily adaptable for commercial preparation of (-)-trans-4-(4-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine of formula-(I).本发明揭示了一种用于制备抗抑郁药帕罗西汀的先进中间体(-)-反式-4-(4-氟苯基)-3-羟甲基-1-甲基哌啶的改进和对映具体过程。通过使用手性酸对化合物XX进行拆分,然后对已拆分的胺进行氢化,制备了化合物XXII的方法。将化合物XXII与丙烯酸酯发生迈克尔加成反应,得到了化合物XXIII。将化合物XXIII中的羟基转化为一个脱离基,然后经过强碱处理,得到了对映具体的分子内环化哌啶衍生物XXV。将化合物XXV中的酯基还原为金属氢化物还原剂,得到了具有超过97%对映纯度的化合物I。通过在多种溶剂中进行一次结晶,将化合物I进一步纯化至>99.5%。本过程易于用于商业制备(-)-反式-4-(4-氟苯基)-3-羟甲基-1-甲基哌啶的方法。
-
Substituted phenylpiperidines with serotoninergic activity and enhanced therapeutic properties申请人:Gant G. Thomas公开号:US20070112031A1公开(公告)日:2007-05-17Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and/or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and/or premature ejaculation are described.
-
[EN] NOVEL PROCESS FOR THE PREPARATION OF 4-ARYL-3-HYDROXYMETHYL-1-METHYLPIPERIDINES.<br/>[FR] NOUVEAU PROCEDE DE PREPARATION DE 4-ARYL-3-HYDROXYMETHYL-1-METHYLPIPERIDINES申请人:NATCO PHARMA LTD公开号:WO2004043921A1公开(公告)日:2004-05-27A novel, improved, and general process for the preparation of 4-aryl-3-hydroxymethyl-1-methylpiperidines is disclosed in the present invention. 4-(4-Fluorophenyl)-3-hydroxymethyl-1-methylpiperidine is a well-known intermediate in making the anti-depressant drug, paroxetine ((-)-trans-4-p-fluorophenyl-3-(3',4'-methylenedioxy-phenoxymethyl)piperidine). Novel N-methyl-N-[3-(4-substitutedphenyl (F, Me, OMe))-3-hydroxy]propylamines are prepared from the Mannich salts such as 3-dimethylamino- or 3-(N-methyl-N-benzylamino)-4'-substituted (F, Me, OMe) propiophenone hydrochlorides by conventional methods. The N-methyl-N-[3-(4-substitutedphenyl (H, F, Me, OMe))-3-hydroxy]propylamines thus obtained are reacted with ethyl or methyl acrylate to get the corresponding Michael addition products. The hydroxy group present in the Michael addition products is converted into a facile leaving group and treated with a strong base to get 4-aryl-N-methylpiperidine-3-carboxylates via the intramolecular cyclization in good yields. Reduction of the ester group present in these piperidine-3-carboxylates gave the title compounds as crystalline solids. Present process is easily adaptable for commercial preparation of the paroxetine intermediate (4-(4-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine).本发明揭示了一种新颖的、改进的、通用的制备4-芳基-3-羟甲基-1-甲基哌啶的方法。4-(4-氟苯基)-3-羟甲基-1-甲基哌啶是制造抗抑郁药物帕罗西汀((-)-trans-4-p-氟苯基-3-(3',4'-亚甲二氧基苯氧甲基)哌啶)的已知中间体。新型的N-甲基-N-[3-(4-取代苯基(F,Me,OMe))-3-羟基]丙胺可通过常规方法从Mannich盐制备而来,例如3-二甲氨基或3-(N-甲基-N-苄基氨基)-4'-取代(F,Me,OMe)丙酮盐酸盐。因此获得的N-甲基-N-[3-(4-取代苯基(H,F,Me,OMe))-3-羟基]丙胺与乙基或甲基丙烯酸酯反应,得到相应的Michael加成产物。Michael加成产物中存在的羟基转化为容易离去的基团,并用强碱处理,通过分子内环化以良好的产率得到4-芳基-N-甲基哌啶-3-羧酸酯。这些哌啶-3-羧酸酯中存在的酯基还原后,得到了晶体固体的目标化合物。目前的方法易于用于商业制备帕罗西汀中间体(4-(4-氟苯基)-3-羟甲基-1-甲基哌啶)。
-
PREPARATION OF PAROXETINE HYDROCHLORIDE HEMIHYDRATE
-
[EN] AN IMPROVED PROCESS FOR MINIMISING THE FORMATION OF DEHALOGENATED BYPRODUCTS<br/>[FR] PROCÉDÉ AMÉLIORÉ PERMETTANT DE RÉDUIRE AU MINIMUM LA FORMATION DE SOUS-PRODUITS DÉSHALOGÉNÉS申请人:PIRAMAL ENTPR LTD公开号:WO2015071831A1公开(公告)日:2015-05-21The present invention provides an improved process for preparation of organic compounds represented by Formula-Z; wherein effectively minimising the formation of dehalogenated by-products is achieved. In the process, the reduction is carried out using suitable reducing agent; more preferably Lithium Aluminium Hydride (LAH) in a solvent system, wherein at least one of the solvent is selected from halogenated solvents, which acts as co-solvent. The process of the present invention is useful for minimising of the formation of dehalogenated by-products during synthesis of various active pharmaceutical ingredients such as Paroxetine Hydrochloride, Cinacalcet, Eletriptan and Asenapine.
表征谱图
-
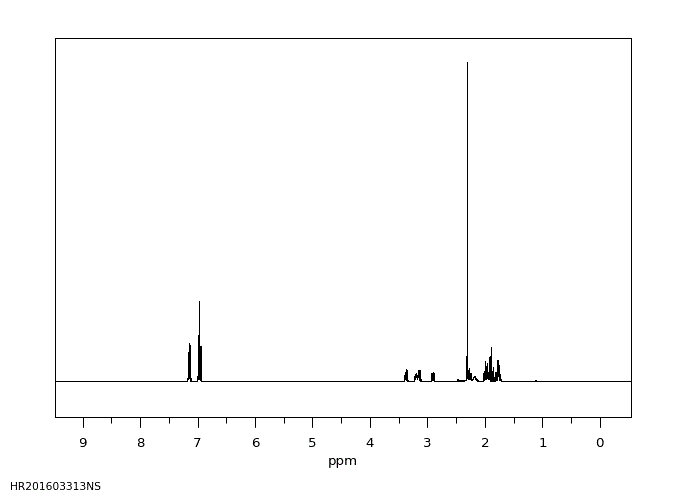
氢谱1HNMR
-
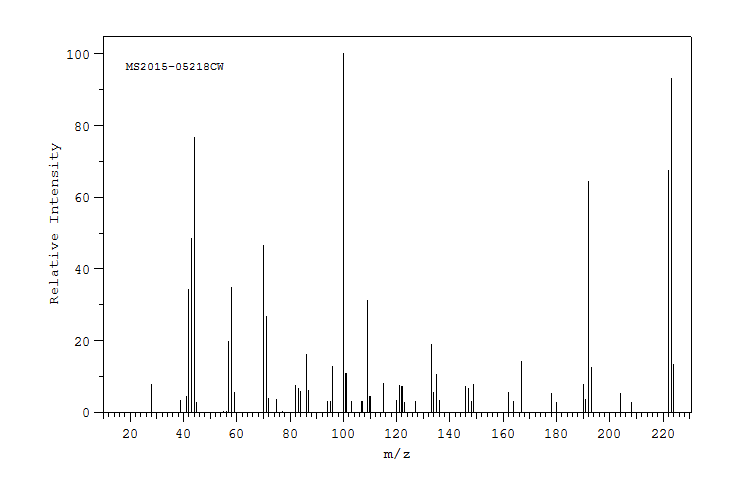
质谱MS
-
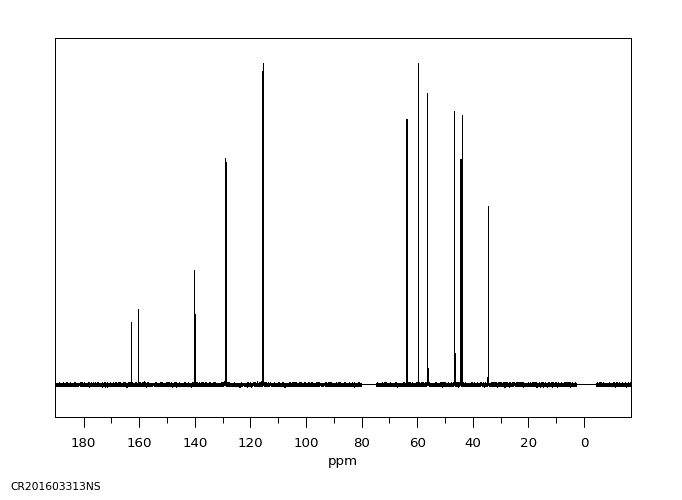
碳谱13CNMR
-
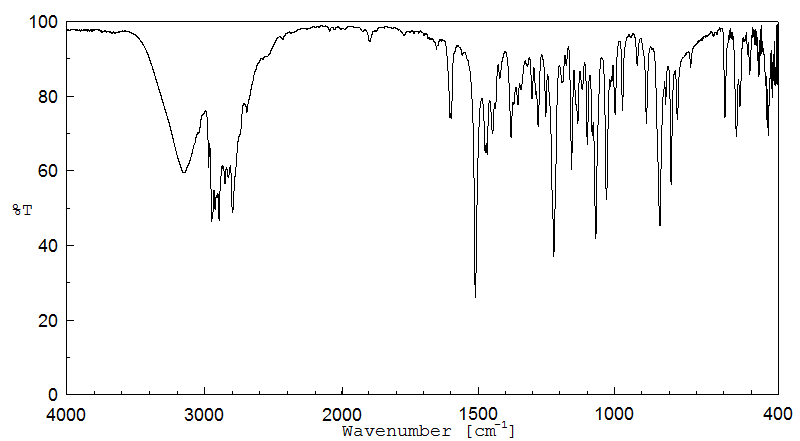
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-甲基哌啶盐酸盐;
(R)-2-苄基哌啶-1-羧酸叔丁酯
((3S,4R)-3-氨基-4-羟基哌啶-1-基)(2-(1-(环丙基甲基)-1H-吲哚-2-基)-7-甲氧基-1-甲基-1H-苯并[d]咪唑-5-基)甲酮盐酸盐
高氯酸哌啶
高托品酮肟
马来酸帕罗西汀
颜料红48:4
顺式3-氟哌啶-4-醇盐酸盐
顺式2,6-二甲基哌啶-4-酮
顺式1-苄基-4-甲基-3-甲氨基-哌啶
顺式-叔丁基4-羟基-3-甲基哌啶-1-羧酸酯
顺式-6-甲基-哌啶-1,3-二甲酸1-叔丁酯
顺式-5-(三氟甲基)哌啶-3-羧酸甲酯盐酸盐
顺式-4-叔丁基-2-甲基哌啶
顺式-4-Boc-氨基哌啶-3-甲酸甲酯
顺式-4-(氮杂环丁烷-1-基)-3-氟哌
顺式-3-顺式-4-氨基哌啶
顺式-3-甲氧基-4-氨基哌啶
顺式-3-BOC-3,7-二氮杂双环[4.2.0]辛烷
顺式-3-(1-吡咯烷基)环丁腈
顺式-3,5-哌啶二羧酸
顺式-3,4-二溴-3-甲基吡咯烷盐酸盐
顺式-2,6-二甲基-4-氧代哌啶-1-羧酸叔丁基酯
顺式-1-叔丁氧羰基-4-甲基氨基-3-羟基哌啶
顺式-1-boc-3,4-二氨基哌啶
顺式-1-(4-叔丁基环己基)-4-苯基-4-哌啶腈
顺式-1,3-二甲基-4-乙炔基-6-苯基-3,4-哌啶二醇
顺-4-(4-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-4-(2-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-3-氨基-4-氟哌啶-1-羧酸叔丁酯
顺-1-苄基-4-甲基哌啶-3-氨基酸甲酯盐酸盐
非莫西汀
雷芬那辛
雷拉地尔
阿维巴坦中间体4
阿格列汀杂质
阿尼利定盐酸盐 CII
阿尼利定
阿塔匹酮
阿哌沙班杂质BMS-591455
阿哌沙班杂质87
阿哌沙班杂质52
阿哌沙班杂质51
阿哌沙班杂质5
阿哌沙班杂质
阿哌沙班杂质
阿哌沙班-d3
阿哌沙班
阻聚剂701
间氨基谷氨酰胺