1,2-di(naphthalen-2-yl)diselane | 17932-23-9
中文名称
——
中文别名
——
英文名称
1,2-di(naphthalen-2-yl)diselane
英文别名
bis(2-naphthalenyl) diselenide;2-naphthyl diselenide;Diselenide, di-2-naphthalenyl;2-(naphthalen-2-yldiselanyl)naphthalene
CAS
17932-23-9
化学式
C20H14Se2
mdl
——
分子量
412.251
InChiKey
YIFOZOFOZLPSSW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.27
-
重原子数:22
-
可旋转键数:3
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
反应信息
-
作为反应物:参考文献:名称:铜 (I) 催化的共轭加成/与硒醇和 α-取代的 α,β-不饱和硫代酰胺的对映选择性质子化摘要:公开了铜 (I) 催化的与硒醇和 α-取代的 α,β-不饱和硫代酰胺的共轭加成/对映选择性质子化,它提供了一系列具有高对映选择性的手性硒化物。DOI:10.1002/anie.202301422
-
作为产物:描述:2-碘萘 在 selenium 、 copper(II) oxide 、 potassium hydroxide 作用下, 以 二甲基亚砜 为溶剂, 生成 1,2-di(naphthalen-2-yl)diselane参考文献:名称:通过脱羧偶联反应区域选择性合成硒醚摘要:已开发出一种有效的,选择性的方法,可以从容易获得的二硒化物和苯乙酸合成含一个或两个双键C-Se键的硒醚。通过在无金属条件下将空气用作氧化剂来制备包含一个C-Se键的化合物,而通过氯化铁(III)/氧/碳酸铯(FeCl 3 / O 2)形成具有两个双键C-Se键的化合物。/ Cs 2 CO 3)系统。此外,在标准反应条件下,1,2-二苯基二硫烷也可以平稳地转化为相应的硫醚产物。DOI:10.1002/adsc.201700676
文献信息
-
Iodosobenzene-Mediated Three-Component Selenofunctionalization of Olefins作者:Zhi-Peng Liang、Wei Yi、Peng-Fei Wang、Gong-Qing Liu、Yong LingDOI:10.1021/acs.joc.1c00257日期:2021.4.2A three-component reaction of olefin, diselenide and water, alcohols, phenol, carboxylic acid, or amine by a commercially available hypervalent iodine(III) reagent, PhIO, was developed. This method provides access to a wide range of vicinally functionalized selenoderivatives under ambient conditions with mostly excellent yields and high diastereoselectivity. The developed reaction displays high levels
-
New synthetic methods : sodium alkanechalcogenates as demethylating agents. Scope, limitation and new one-pot synthesis of diaryldiselenides.作者:Michel Evers、Léon ChristiaensDOI:10.1016/s0040-4039(00)81412-4日期:——Sodium alkanechalcogenates (S, Se) cleave the alkylarylchalcogenides (O, S, Se). The versatility of such reagents is developed and applied to a new synthesis of diaryldiselenides.
-
Visible-light-induced oxidative coupling of vinylarenes with diselenides leading to α-aryl and α-alkyl selenomethyl ketones作者:Gong-Qing Liu、Wei Yi、Peng-Fei Wang、Ji Liu、Meng Ma、Da-Yun Hao、Liang Ming、Yong LingDOI:10.1039/d1gc00049g日期:——A visible-light-induced oxidative coupling of diselenides with readily available vinylarenes is demonstrated. This benign protocol allows one to access a wide range of α-aryl and α-alkyl selenomethyl ketones in good yields with excellent functional group compatibility. The distinct advantages of this protocol over all previous methods include the use of a green solvent and air as an oxidant and the
-
Glycerol as a promoting medium for cross-coupling reactions of diaryl diselenides with vinyl bromides作者:Loren C. Gonçalves、Gabriela F. Fiss、Gelson Perin、Diego Alves、Raquel G. Jacob、Eder J. LenardãoDOI:10.1016/j.tetlet.2010.10.107日期:2010.12herein the use of glycerol as a novel solvent in the cross-coupling reaction of diaryl diselenides with vinyl bromides catalyzed by CuI. This cross-coupling reaction was performed with diaryl diselenides and (Z)- or (E)-vinyl bromides bearing electron-withdrawing and electron-donating groups, affording the corresponding vinyl selenides in good to excellent yields. The mixture glycerol/catalyst can be
-
Atom-economical selenation of electron-rich arenes and phosphonates with molecular oxygen at room temperature
表征谱图
-
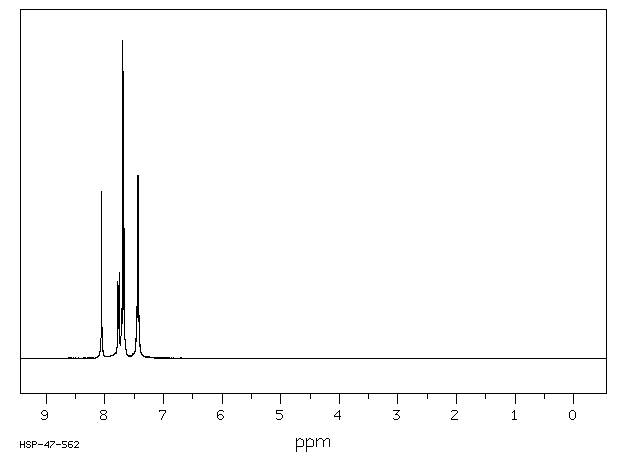
氢谱1HNMR
-
质谱MS
-
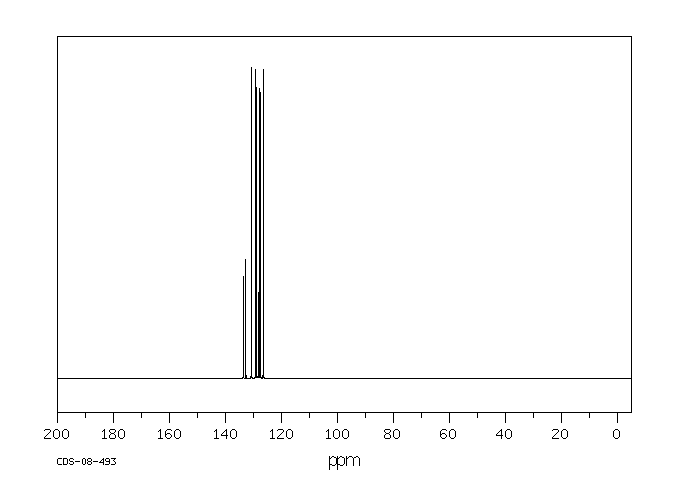
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮