苯基三乙氧基硅烷 | 780-69-8
中文名称
苯基三乙氧基硅烷
中文别名
三乙氧基苯基硅烷;苯基三乙氧基
英文名称
Phenyltriethoxysilan
英文别名
triethoxyphenylsilane;triethoxyphenylsilan;phenyltriethoxysilane;triethoxy(phenyl)silane
CAS
780-69-8
化学式
C12H20O3Si
mdl
MFCD00009065
分子量
240.374
InChiKey
JCVQKRGIASEUKR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:112-113 °C10 mm Hg(lit.)
-
密度:0.996 g/mL at 25 °C(lit.)
-
蒸气密度:>1 (vs air)
-
闪点:109 °F
-
LogP:-0.31-3.4 at 22℃
-
物理描述:Liquid
-
保留指数:1298.8
-
稳定性/保质期:
在常温常压下保持稳定,应避免与强氧化剂和水分直接接触。
计算性质
-
辛醇/水分配系数(LogP):3.25
-
重原子数:16
-
可旋转键数:7
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:27.7
-
氢给体数:0
-
氢受体数:3
安全信息
-
TSCA:Yes
-
危险等级:3.2
-
危险品标志:Xn
-
安全说明:S16,S24/25,S26,S37/39
-
危险类别码:R10,R21
-
WGK Germany:3
-
海关编码:29310095
-
危险品运输编号:UN 1993 3/PG 3
-
危险类别:3.2
-
RTECS号:VV4900000
-
包装等级:III
-
危险标志:GHS02
-
危险性描述:H226
-
危险性防范说明:P210,P260,P370+P378
-
储存条件:储存于干燥的惰性气体中,并保持容器密封。将其存放在阴凉、干燥处。
SDS
| Name: | Phenyltriethoxysilane 98% Material Safety Data Sheet |
| Synonym: | Triethoxyphenylsilane |
| CAS: | 780-69-8 |
Synonym:Triethoxyphenylsilane
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 780-69-8 | Phenyltriethoxysilane | 98 | 212-305-8 |
Risk Phrases: 10 21
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable. Harmful in contact with skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. Ingestion of large amounts may cause CNS depression.
Inhalation:
Causes respiratory tract irritation.
Chronic:
Ethanol is mainly metabolized in the liver, which is also one of the primary target organs. While ethanol is well known to cause cirrhosis of the liver in alcoholics, liver cirrhosis has also been produced in rabbits exposed by inhalation.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid. Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air.
Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Flammable liquid and vapor. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Contact with water liberates highly flammable gases.
Extinguishing Media:
Use carbon dioxide or dry chemical. Do NOT get water inside containers.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation. Do not get water inside containers. A vapor suppressing foam may be used to reduce vapors.
Section 7 - HANDLING and STORAGE
Handling:
Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame. Avoid ingestion and inhalation. Use with adequate ventilation. Do not allow contact with water. Wash clothing before reuse. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames. Keep from contact with moist air and steam.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Flammables-area. Store protected from moisture.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 780-69-8: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use. Wear a NIOSH/MSHA or European Standard EN 149 approved full-facepiece airline respirator in the positive pressure mode with emergency escape provisions.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear, colorless
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 112-113 deg C @ 10 mm Hg
Freezing/Melting Point: Not available.
Autoignition Temperature: 265 deg C ( 509.00 deg F)
Flash Point: 42 deg C ( 107.60 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Reacts.
Specific Gravity/Density: .9900 g/ml
Molecular Formula: C12H20O3Si
Molecular Weight: 240.38
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions. Material generates ethanol on contact with water.
Conditions to Avoid:
Ignition sources, moisture, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong acids, water.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, silicon dioxide, ethanol.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 780-69-8: VV4900000 LD50/LC50:
CAS# 780-69-8: Draize test, rabbit, eye: 500 mg/24H Mild; Draize test, rabbit, skin: 500 mg/24H Mild; Oral, rat: LD50 = 2830 uL/kg; Skin, rabbit: LD50 = 3180 uL/kg.
Carcinogenicity:
Phenyltriethoxysilane - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: FLAMMABLE LIQUID, N.O.S.*
Hazard Class: 3
UN Number: 1993
Packing Group: III
IMO
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3.3
UN Number: 1993
Packing Group: III
RID/ADR
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3
UN Number: 1993
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 10 Flammable.
R 21 Harmful in contact with skin.
Safety Phrases:
S 16 Keep away from sources of ignition - No
smoking.
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 780-69-8: No information available.
Canada
CAS# 780-69-8 is listed on Canada's DSL List.
CAS# 780-69-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 780-69-8 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯基三甲氧基硅烷 phenyl trimethylsiloxane 2996-92-1 C9H14O3Si 198.294 氯二乙氧基苯基硅烷 phenyldiethoxychlorosilane 17903-53-6 C10H15ClO2Si 230.766 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 三(2-氨基乙氧基)苯基硅烷 Tri-(β-aminoaethoxy)-phenylsilan 17146-69-9 C12H23N3O3Si 285.418 —— phenyltripropoxysilane 17902-99-7 C15H26O3Si 282.455 —— phenyltri(n-butoxy)silane 10581-02-9 C18H32O3Si 324.536 —— phenyltri(octoxy)silane 13340-44-8 C30H56O3Si 492.858 苯基三甲氧基硅烷 phenyl trimethylsiloxane 2996-92-1 C9H14O3Si 198.294 1-苯基-2,8,9-三氧杂-5-氮杂-1-硅杂二环[3.3.3]十一烷 1-phenyl-2,8,9-trioxa-5-aza-1-silabicyclo{3.3.3}undecane 2097-19-0 C12H17NO3Si 251.357 —— 1-phenyl-2,9,10-trioxa-6-aza-1-sila-bicyclo[4.3.3]dodecane 69656-40-2 C13H19NO3Si 265.384 氯二乙氧基苯基硅烷 phenyldiethoxychlorosilane 17903-53-6 C10H15ClO2Si 230.766 —— 1-phenylsilatranone 53883-47-9 C12H15NO4Si 265.341
反应信息
-
作为反应物:参考文献:名称:CARBON–SILICON BOND CLEAVAGE OF ORGANOTRIALKOXYSILANES AND ORGANOSILATRANES WITHm-CHLOROPERBENZOIC ACID ANDN-BROMOSUCCINIMIDE. NEW ROUTE TO PHENOLS, PRIMARY ALCOHOLS AND BROMIDES摘要:烷基和芳基三乙氧基硅烷能够顺利地通过间氯过氧苯甲酸(m-氯过氧苯甲酸)进行氧化碳硅键断裂,得到相应的醇。类似地,硅拉烷分别与m-氯过氧苯甲酸和N-溴代丁二酰亚胺反应,分别得到醇和溴化物。DOI:10.1246/cl.1981.243
-
作为产物:参考文献:名称:Khotinsky, E.; Seregenkoff, B., Archives des Sciences Physiques et Naturelles, 1908, vol. 25, p. 516 - 516摘要:DOI:
-
作为试剂:参考文献:名称:[DE] SUBSTITUIERTE CYCLOALKANE UND IHRE VERWENDUNG ALS INITIATOREN ZUR KATIONISCHEN POLYMERISATION
[EN] SUBSTITUTED CYCLOALKANES, AND USE THEREOF AS CATIONIC POLYMERIZATION INITIATORS
[FR] CYCLOALCANES SUBSTITUES ET LEUR UTILISATION COMME INITIATEURS POUR LA POLYMERISATION CATIONIQUE摘要:描述了取代的7至12元环烷烃,其在三级环烷碳原子上具有离去基团,特别是氯原子,以及它们的制备方法和作为阳离子聚合引发剂的用途,特别是用于异丁烯的阳离子聚合。首选化合物是1,4-二氯-1,4-二甲基环辛烷,1,5-二氯-1,5-二甲基环辛烷及其混合物。它们是通过将氯化氢加到适当取代的环烷烯烃上制备的。公开号:WO2005044766A1
文献信息
-
Valorisation of urban waste to access low-cost heterogeneous palladium catalysts for cross-coupling reactions in biomass-derived γ-valerolactone作者:Federica Valentini、Francesco Ferlin、Simone Lilli、Assunta Marrocchi、Liu Ping、Yanlong Gu、Luigi VaccaroDOI:10.1039/d1gc01707a日期:——commercial Pd/C catalyst. The good reactivity in the biomass-derived solvent (GVL) confirms that the Pd/PiNe heterogeneous catalyst is a valid system that can be integrated into a waste valorization chain within a circular economy approach. In addition, the efficiency of the catalyst has also been extended to perform the challenging consecutive Hiyama–Heck reaction to afford differently substituted (E)-1在这里,我们报告了一个简单的协议,用于评估普通城市生物废物的价值。将松针城市垃圾预处理后获得的木质纤维素生物质有效地转化为用于固定 Pd 纳米颗粒的低成本载体 (PiNe)。最终的 Pd/PiNe 多相催化剂具有小粒径 (4.5 nm) 和金属负载 (9.9 wt%),可与大多数市售和常用的对应物相媲美。在这项贡献中,我们测试了 Pd/PiNe 系统在两个代表性交叉偶联 Heck 和 Hiyama 反应中的催化效率,并比较了与商业 Pd/C 催化剂获得的结果。生物质衍生溶剂 (GVL) 中的良好反应性证实了 Pd/PiNe 多相催化剂是一种有效的系统,可以在循环经济方法中整合到废物价值链中。此外,催化剂的效率也得到了扩展,以进行具有挑战性的连续 Hiyama-Heck 反应,以提供不同取代的(E )-1,2-二芳基乙烯。
-
Synthesis of Esters by Functionalisation of CO2申请人:Commissariat a L'Energie Atomique et aux Energies Alternatives公开号:US20170240485A1公开(公告)日:2017-08-24The invention relates to a method for (I) producing a carboxylic ester of formula (I). Said method comprises the steps of: a) bringing an organosilane/borane of formula Si or B into contact with CO 2 , in the presence of a catalyst and an electrophilic compound of formula (III), the groups R 1 , R 2 , R 3 , R 4 , R 5 , Y, and M′ being as defined in claim 1; and optionally b) recovering the compound of formula (I) produced.
-
Rhodium(III)-Catalyzed Direct C–H Arylation of Various Acyclic Enamides with Arylsilanes作者:Xiaolan Li、Kai Sun、Wenjuan Shen、Yong Zhang、Ming-Zhu Lu、Xuzhong Luo、Haiqing LuoDOI:10.1021/acs.orglett.0c03578日期:2021.1.1The stereoselective β-C(sp2)–H arylation of various acyclic enamides with arylsilanes via Rh(III)-catalyzed cross-coupling reaction was illustrated. The methodology was characterized by extraordinary efficacy and stereoselectivity, a wide scope of substrates, good functional group tolerance, and the adoption of environmentally friendly arylsilanes. The utility of this present method was evidenced by
-
Bench-Stable Cobalt Pre-Catalysts for Mild Hydrosilative Reduction of Tertiary Amides to Amines and Beyond作者:Alibek Nurseiit、Jaysan Janabel、Kristina A. Gudun、Aishabibi Kassymbek、Medet Segizbayev、Tulegen M. Seilkhanov、Andrey Y. KhalimonDOI:10.1002/cctc.201801605日期:2019.1.23unsaturated organic molecules, including the normally challenging reduction of amides to amines. With regard to hydrosilative reduction of amides even more effective and activator free catalytic systems can be generated from the bench‐stable, commercially available Co(acac)2 and Co(OAc)2 with dpephos and PPh3 ligands. These systems operate under mild conditions (<100 °C), with many examples of room temperature
-
Pd-catalyzed cross-coupling reactions of arenediazonium salts with arylsilanes and aryltrifluoroborates in water作者:Kai Cheng、Baoli Zhao、Sai Hu、Xian-Man Zhang、Chenze QiDOI:10.1016/j.tetlet.2013.09.001日期:2013.11Pd-catalyzed cross-coupling reactions of various arenediazonium salts with ArSi(OR)3 and KArBF3 have been achieved in good to excellent yields under simple aerobic conditions in water at room temperature. The functional group tolerance makes these transformations as attractive alternatives to the traditional cross-coupling approaches. Furthermore, the sequence can also be performed in a one-pot domino
表征谱图
-
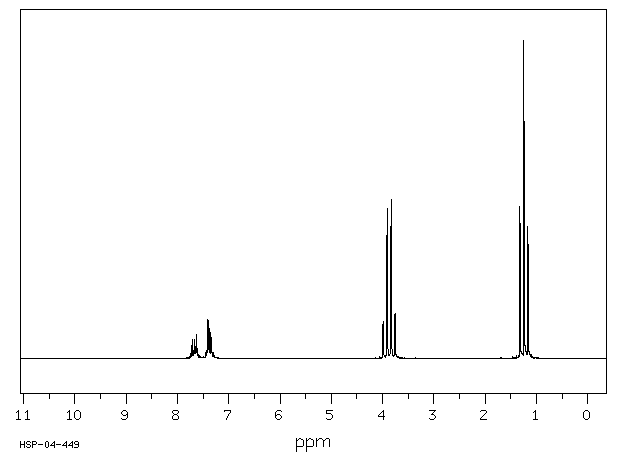
氢谱1HNMR
-
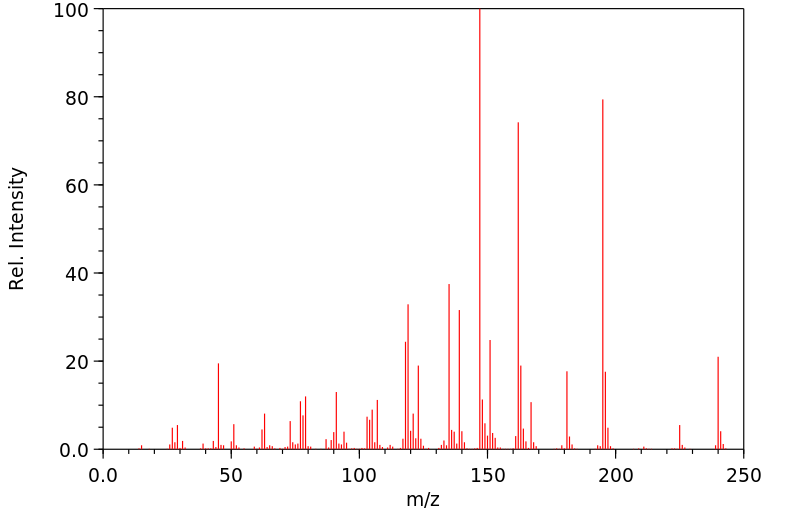
质谱MS
-
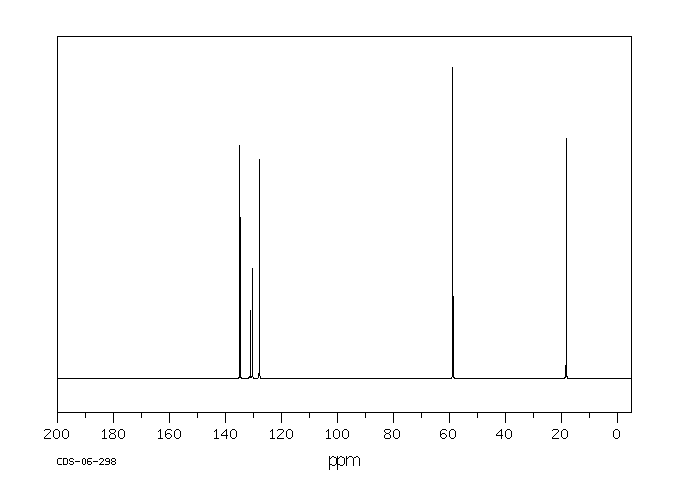
碳谱13CNMR
-
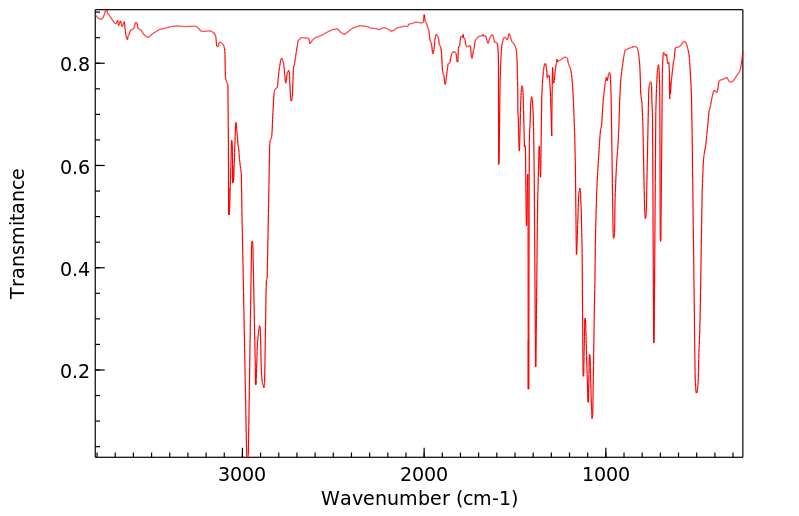
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-溴乙氧基)-特丁基二甲基硅烷
鲸蜡基聚二甲基硅氧烷
骨化醇杂质DCP
马沙骨化醇中间体
马来酸双(三甲硅烷)酯
顺式-二氯二(二甲基硒醚)铂(II)
顺-N-(1-(2-乙氧基乙基)-3-甲基-4-哌啶基)-N-苯基苯酰胺
降钙素杂质13
降冰片烯基乙基三甲氧基硅烷
降冰片烯基乙基-POSS
间-氨基苯基三甲氧基硅烷
镓,二(1,1-二甲基乙基)甲基-
镁,氯[[二甲基(1-甲基乙氧基)甲硅烷基]甲基]-
锑,二溴三丁基-
铷,[三(三甲基甲硅烷基)甲基]-
铂(0)-1,3-二乙烯-1,1,3,3-四甲基二硅氧烷
钾(4-{[二甲基(2-甲基-2-丙基)硅烷基]氧基}-1-丁炔-1-基)(三氟)硼酸酯(1-)
金刚烷基乙基三氯硅烷
酰氧基丙基双封头
达格列净杂质
辛醛,8-[[(1,1-二甲基乙基)二甲基甲硅烷基]氧代]-
辛甲基-1,4-二氧杂-2,3,5,6-四硅杂环己烷
辛基铵甲烷砷酸盐
辛基衍生化硅胶(C8)ZORBAX?LP100/40C8
辛基硅三醇
辛基甲基二乙氧基硅烷
辛基三甲氧基硅烷
辛基三氯硅烷
辛基(三苯基)硅烷
辛乙基三硅氧烷
路易氏剂-3
路易氏剂-2
路易士剂
试剂Cyanomethyl[3-(trimethoxysilyl)propyl]trithiocarbonate
试剂3-[Tris(trimethylsiloxy)silyl]propylvinylcarbamate
试剂3-(Trimethoxysilyl)propylvinylcarbamate
试剂2-(Trimethylsilyl)cyclopent-2-en-1-one
试剂11-Azidoundecyltriethoxysilane
西甲硅油杂质14
衣康酸二(三甲基硅基)酯
苯胺,4-[2-(三乙氧基甲硅烷基)乙基]-
苯磺酸,羟基-,盐,单钠聚合甲醛,1,3,5-三嗪-2,4,6-三胺和脲
苯甲醇,a-[(三苯代甲硅烷基)甲基]-
苯并磷杂硅杂英,5,10-二氢-10,10-二甲基-5-苯基-
苯基二甲基氯硅烷
苯基二甲基乙氧基硅
苯基二甲基(2'-甲氧基乙氧基)硅烷
苯基乙酰氧基三甲基硅烷
苯基三辛基硅烷
苯基三甲氧基硅烷