butanal O-methyloxime | 31376-98-4
中文名称
——
中文别名
——
英文名称
butanal O-methyloxime
英文别名
butyraldehyde O-methyl-oxime;n-Butyraldoxim-O-methylaether;n-Butyraldehyd-O-methyloxim;Butanal-O-methyloxim;Butyraldehyde O-methyl oxime;N-methoxybutan-1-imine
CAS
31376-98-4
化学式
C5H11NO
mdl
——
分子量
101.148
InChiKey
BMDFNHAUSSAITP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:107.3±23.0 °C(Predicted)
-
密度:0.82±0.1 g/cm3(Predicted)
-
保留指数:680
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:7
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:21.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2928000090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 丁醛肟 butyraldehyde oxime 110-69-0 C4H9NO 87.1216
反应信息
-
作为反应物:描述:参考文献:名称:Preparation of 2-alkoxyiminoalkyl bromides by the bromination of O-alkyl oximes with N-bromosuccinimide摘要:DOI:10.1021/jo00821a046
-
作为产物:描述:参考文献:名称:Silver ion α-oxy carboxylate-oxime complexes摘要:一种非羟基溶剂可溶性银络合物,包括与α-羟基羧酸酯和肟化合物络合的可还原银离子。该非羟基溶剂可溶性银络合物可以用以下公式(I)表示:(Ag+)a(L)b(P)c (I),其中L代表α-羟基羧酸酯;P代表肟化合物;a为1或2;b为1或2;c为1、2、3或4,但当a为1时,b为1,当a为2时,b为2。这种络合物可以被纳入感光组合物中,通过方法在各种物品上提供薄膜或图案,以提供具有电导性的银金属。公开号:US09783553B1
文献信息
-
Electroreductive intermolecular coupling of ketones with O-methyl oximes. A convenient route to synthesis of 2-amino alcohols作者:Tatsuya Shono、Naoki Kise、Taku FujimotoDOI:10.1016/s0040-4039(00)79486-x日期:1991.1The electroreduction of ketones together with O-methyl oximes gave intermolecularly coupled products, 2-methoxyamino alcohols, which were easily reduced to 2-amino alcohols.
-
Removable Silyl Group as a “Masked Proton” in Oxy-2-oxonia(azonia)-Cope Rearrangement: Applications in Stereoselective Total Synthesis of Natural Macrolides作者:Wenbo Mu、Yue Zou、Lijun Zhou、Quanrui Wang、Andreas GoekeDOI:10.1002/ejoc.201500654日期:2015.8In the presence of a Lewis acid, trimethylsilyl-substituted β,γ-unsaturated ketones and aldehydes (imines) undergo nucleophilic addition to produce zwitterionic intermediates, followed by oxy-2-oxonia(azonia)-Cope rearrangements to give homoallylic esters (amides). In the case of TMS-containing 2-vinylcycloalkanones, the process results in ring-enlargement, providing 10- to 16-membered lactones. This
-
Electroorganic Chemistry. 144. Electroreductive Coupling of Ketones with O-Methyl Oximes, N,N-Dimethylhydrazones, and Nitrones. A Convenient Route to Synthesis of .beta.-Amino Alcohol作者:Tatsuya Shono、Naoki Kise、Taku Fujimoto、Ayuko Yamanami、Ryoji NomuraDOI:10.1021/jo00086a023日期:1994.4The intermolecular coupling of a variety of ketones with some types of O-methyl oximes took place when a mixture of both components was electrochemically reduced in i-PrOH with an Sn cathode. The product, beta-methoxyamino alcohol was easily converted to beta-amino alcohol by simple reduction. A chiral ligand effective for the enantioselective addition of diethylzinc to an aldehyde was easily obtained from the product formed by the electroreductive coupling of (-)-menthone with O-methylacetaldoxime. The intermolecular coupling of a ketone with a N,N-dimethylhydrazone or nitrone was also promoted by the electroreduction. Furthermore, the electroreductive coupling of a carbonyl group with an intramolecular O-methyl oxime moiety gave the corresponding cyclized product stereoselectively.
表征谱图
-
氢谱1HNMR
-
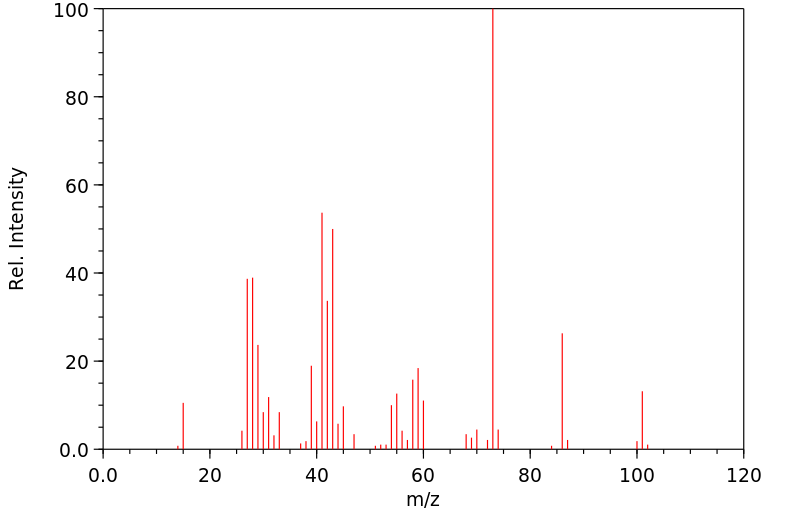
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷