3-氨基-5-甲基异恶唑 | 1072-67-9
中文名称
3-氨基-5-甲基异恶唑
中文别名
3-氨基-5-甲基异唑;5-甲基-3-异恶唑胺;5-甲基邻甲氧基苯胺;3-氨基-5-甲基异噁唑
英文名称
3-Amino-5-methylisoxazole
英文别名
5-methylisoxazol-3-ylamine;5-methylisoxazol-3-amine;5-methyl-1,2-oxazol-3-amine
CAS
1072-67-9
化学式
C4H6N2O
mdl
MFCD00003155
分子量
98.1044
InChiKey
FKPXGNGUVSHWQQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:59-61 °C(lit.)
-
沸点:183.6°C (rough estimate)
-
密度:1.1890 (rough estimate)
-
溶解度:可溶于水
-
稳定性/保质期:
常温常压下稳定,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:52
-
氢给体数:1
-
氢受体数:3
安全信息
-
安全说明:S16,S22,S24/25,S26,S26/37/39/47,S37/39,S47
-
危险品运输编号:NONH for all modes of transport
-
WGK Germany:3
-
海关编码:29349990
-
危险品标志:F
-
危险类别码:R5,R36/37/38,R11
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将容器密封保存,并存放在阴凉、干燥处。
SDS
模块 1. 化学品
1.1 产品标识符
: 3-氨基-5-甲基异噁唑
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C4H6N2O
分子式
: 98.1 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
避免粉尘生成。 避免吸入蒸气、烟雾或气体。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
联合国运输名称: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
联合国运输名称: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所选择身体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 淡黄
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 59 - 61 °C - lit.
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 16. 其他信息
进一步信息
版权所有:2012 Co. LLC. 公司。许可无限制纸张拷贝,仅限于内部使用。
上述信息视为正确,但不包含所有的信息,仅作为指引使用。本文件中的信息是基于我们目前所知,就正
确的安全提示来说适用于本品。该信息不代表对此产品性质的保证。
参见发票或包装条的反面。
模块 15 - 法规信息
N/A
制备方法与用途
3-氨基-5-甲基异恶唑是生产广谱抗生素新诺明的重要中间体,呈现白色晶体状。其熔点为51.5℃,沸点235℃,能溶于醇和醚,并可随水蒸气挥发。该化合物主要用于医药领域,作为磺胺类药物的合成原料。
新诺明是一种广谱抗生素,属于一级抗菌剂,具有结晶性粉末状外观,无臭味且微苦。它几乎不溶于水,但易溶于稀盐酸、氢氧化钠溶液或氨溶液中;熔点为168℃至172℃。临床主要用于治疗尿路感染、呼吸道感染、皮肤化脓性感染、扁桃体炎等疾病,但对磺胺过敏者及严重肝肾患者应避免使用。
新诺明的抗菌谱与SD相似,但在抗菌作用上更为强大。其体内代谢产物乙酰化物溶解度较低,在尿道中容易析出结晶,导致结晶尿、血尿及闭尿等症状;因此,大剂量使用时宜与碳酸氢钠同服。若与增效剂联合应用,则其抗菌效能会显著增强,可增加数倍至数十倍。
临床用途包括治疗扁桃体炎、急性支气管炎、肺部感染、尿路感染、皮肤化脓性感染、菌痢及伤寒等疾病。
化学性质3-氨基-5-甲基异恶唑为白色晶体,熔点为51.5℃,沸点235℃。该化合物溶于醇和醚,并能随水蒸气挥发。
用途作为医药中间体,用于生产磺胺类药物。
生产方法通过5-甲基异噁唑-3-甲酰胺的降解制备而成。具体步骤为:在23℃以下将定量氯通入1.02倍体积的氢氧化钠溶液中,生成次氯酸钠溶液;随后,在20-25℃下将5-甲基异噁唑-3-甲酰胺加入次氯酸钠溶液中反应2小时。反应液用氢氧化钠溶液调节至碱浓度为5.6%,然后以1.5L/min的流速通过加热至176℃、内压约为0.75MPa的管道。流出液经氯仿萃取合并,回收氯仿后冷却,最终得到3-氨基-5-甲基异恶唑。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N,5-二甲基-1,2-恶唑-3-胺 N,5-dimethyl-1,2-oxazol-3-amine 55809-40-0 C5H8N2O 112.131 3-氨基-4-溴-5-甲基异噁唑 4-bromo-5-methylisoxazol-3-amine 5819-40-9 C4H5BrN2O 177.0 4-碘-5-甲基异噁唑-3-胺 3-amino-4-iodo-5-methylisoxazole 930-85-8 C4H5IN2O 224.001 5-甲基-3-异噁唑基异硫氰酸 5-methylisoxazolyl-3-isothiocyanate 321309-33-5 C5H4N2OS 140.166 —— 5-methyl-3-isoxazoleisocyanate 55809-54-6 C5H4N2O2 124.099 —— 4-chloro-5-methylisoxazol-3-amine 5819-39-6 C4H5ClN2O 132.549 —— 3-azido-5-methyl-isoxazole —— C4H4N4O 124.102 —— N,N'-methylenebis(5-methylisoxazol-3-amine) 1320357-13-8 C9H12N4O2 208.22
反应信息
-
作为反应物:描述:3-氨基-5-甲基异恶唑 在 N,N-二甲基甲酰胺 氯化亚砜 、 4-二甲氨基吡啶 作用下, 以 二氯甲烷 为溶剂, 反应 1.5h, 以10%的产率得到4-(4-dimethylcarbamoyl-3-fluoro-phenoxy)-2,2-dimethyl-2,3-dihydro-benzofuran-6-carboxylic acid (5-methyl-isoxazol-3-yl)-amide参考文献:名称:WO2007/122482摘要:公开号:
-
作为产物:描述:参考文献:名称:Vivona, Nicolo; Buscemi, Silvestre; Frenna, Vincenzo, Journal of the Chemical Society. Perkin transactions I, 1986, p. 17 - 20摘要:DOI:
-
作为试剂:描述:1,3-二甲基巴比妥酸 、 4-氯苯甲醛 在 3-氨基-5-甲基异恶唑 作用下, 以 乙醇 为溶剂, 生成 5-(4-chlorobenzylidene)-1,3-dimethylbarbituric acid参考文献:名称:涉及3(5)-氨基异恶唑,醛和Meldrum或N,N'-二甲基巴比妥酸衍生物的面向多样性的多组分杂环摘要:在经典和非经典方法(微波和超声波辐射)的帮助下,在芳香族醛与Meldrum或N,N'-二甲基巴比妥酸的多组分杂环化反应中,对5-氨基-3-甲基异恶唑和3-氨基-5-甲基异恶唑进行了详细研究。激活方法。DOI:10.1002/jhet.2656
文献信息
-
Process for the preparation of申请人:Warner-Lambert Company公开号:US03960856A1公开(公告)日:1976-06-01An improved process for the preparation of 4-hydroxy-3-(5-methyl-3-isoxazolylcarbamoyl)-2-methyl-2H-1,2-benzothiazine 1,1-dioxide (I), a known anti-inflammatory agent, requires the use of specific proportions of reactants and carefully controlled reaction conditions. An alkali metal alkoxide, suspended in dimethylformamide is combined, with stirring, as rapidly as possible with a solution of alkyl 2,3-dihydro-3-oxo-1,2-benzisothiazole-2-acetate 1,1-dioxide (II) in dimethylformamide, while maining the internal reaction temperature within 15.degree.-30.degree.C. More than two but less than six moles of the alkoxide are used per mole of the alkyl 2,3-dihydro-3-oxo-1,2-benzisothiazole-2-acetate 1,1-dioxide (II). After all reactants have been combined, stirring is continued for a specific period of time and then the reaction mixture is acidified. Total elapsed time from initial combination of reactants to acidification is from 30 to 50 minutes. Acidification of the reaction mixture precipitates out alkyl 4-hydroxy-2H-1,2-benzothiazine-3-carboxylate 1,1-dioxide (III) in substantially pure form in high yields, without recrystallization. Product III is methylated on the sulfonamide nitrogen and reacted with 3-amino-5-methyl-isoxazole to obtain crude I. A further improvement in the process of the invention involves a more efficient method for purifying crude product I: the need for large quantities of dioxane solvent is obviated. After slurrying and washing, product I is solubilized in dilute alkali, and decolorized. After filtration and acidification pure product I in high yield is obtained. In addition to preparing the known anti-inflammatory agent (I), the initial reaction step of the invention wherein 2,3-dihydro-3-oxo-1,2-benzisothiazole-2-acetate, 1,1-dioxide (II) is rearranged to form alkyl 4-hydroxy-2H-1,2-benzothiazine-3-carboxylate 1,1-dioxide (III), may be used with particular advantage for the preparation of other useful benzothiazine derivatives.一种改进的制备4-羟基-3-(5-甲基-3-异噁唑基甲酰基)-2-甲基-2H-1,2-苯并噻嗪-1,1-二氧化物(I),一种已知的抗炎药物的过程,需要使用特定比例的反应物和精确控制的反应条件。在二甲基甲酰胺中悬浮的碱金属烷氧化物与二甲基甲酰胺中的烷基2,3-二氢-3-酮-1,2-苯并噻嗪-2-乙酸酯1,1-二氧化物(II)的溶液迅速混合,并在保持内部反应温度在15度至30度之间的情况下搅拌。每摩尔烷氧化物使用两个以上但少于六个摩尔的烷基2,3-二氢-3-酮-1,2-苯并噻嗪-2-乙酸酯1,1-二氧化物(II)。在所有反应物混合后,继续搅拌一段特定时间,然后将反应混合物酸化。从初始混合反应物到酸化的总经过时间为30至50分钟。酸化反应混合物使烷基4-羟基-2H-1,2-苯并噻嗪-3-羧酸酯1,1-二氧化物(III)以高收率的基本纯形式沉淀出来,无需再结晶。产品III在磺胺酰氮上甲基化,并与3-氨基-5-甲基-异噁唑发生反应,得到粗I。发明的过程中的进一步改进涉及一种更有效的精制粗产品I的方法:无需大量二噁烷溶剂。在搅拌和洗涤后,产品I在稀碱中溶解,并脱色。经过过滤和酸化后,高收率得到纯产品I。除了制备已知的抗炎药物(I)外,发明的初始反应步骤中,其中2,3-二氢-3-酮-1,2-苯并噻嗪-2-乙酸酯,1,1-二氧化物(II)被重新排列以形成烷基4-羟基-2H-1,2-苯并噻嗪-3-羧酸酯1,1-二氧化物(III),可能特别有利于制备其他有用的苯并噻嗪衍生物。
-
IDO INHIBITORS申请人:BRISTOL-MYERS SQUIBB COMPANY公开号:US20160289171A1公开(公告)日:2016-10-06There are disclosed compounds that modulate or inhibit the enzymatic activity of indoleamine 2,3-dioxygenase (IDO), pharmaceutical compositions containing said compounds and methods of treating proliferative disorders, such as cancer, viral infections and/or inflammatory disorders utilizing the compounds of the invention.已披露的化合物可调节或抑制吲哌酮胺2,3-二氧化酶(IDO)的酶活性,含有该化合物的药物组合物以及利用本发明的化合物治疗增殖性疾病,如癌症、病毒感染和/或炎症性疾病的方法。
-
[EN] NOVEL ANTI-INFLAMMATORY AGENTS<br/>[FR] NOUVEAUX AGENTS ANTI-INFLAMMATOIRES申请人:RESVERLOGIX CORP公开号:WO2010123975A1公开(公告)日:2010-10-28Disclosed are methods of regulating interleukin-6 (IL-6) and/or vascular cell adhesion molecule-1 (VCAM-1) and methods of treating and/or preventing cardiovascular and inflammatory diseases and related disease states, such as, for example, atherosclerosis, asthma, arthritis, cancer, multiple sclerosis, psoriasis, and inflammatory bowel diseases, and autoimmune disease(s) by administering a naturally occurring or synthetic quinazolone derivative. The invention provides novel synthetic quinazolone compounds, as well as pharmaceutical compositions comprising those compounds.
-
Biphenylsulfonamides and derivatives thereof that modulate the activity of endothelin申请人:——公开号:US20020095041A1公开(公告)日:2002-07-18Biphenylsulfonamides and methods for modulating or altering the activity of the endothelin family of peptides are provided. In particular, bicyclic or tricyclic carbon or heterocyclic ring biphenylsulfonamides and methods using these sulfonamides for inhibiting the binding of an endothelin peptide to an endothelin receptor by contacting the receptor with the sulfonamide are provided. Methods for treating endothelin-mediated disorders by administering effective amounts of one or more of these sulfonamides or prodrugs thereof that inhibit or increase the activity of endothelin are also provided.
-
Regioselective, photochemical bromination of aromatic compounds using N-bromosuccinimide作者:Prakash K. Chhattise、A.V. Ramaswamy、Suresh B. WaghmodeDOI:10.1016/j.tetlet.2007.10.126日期:2008.1Regioselective nuclear bromination of aromatic compounds is investigated with N-bromosuccinimide as the brominating agent under UV irradiation to afford the corresponding brominated compounds. The reaction proceeds at ambient temperature (30 ± 2 °C) without any catalyst. In most of the reactions, regioselectively mono-brominated products are obtained in good to high yields. The conversion and selectivity
表征谱图
-
氢谱1HNMR
-
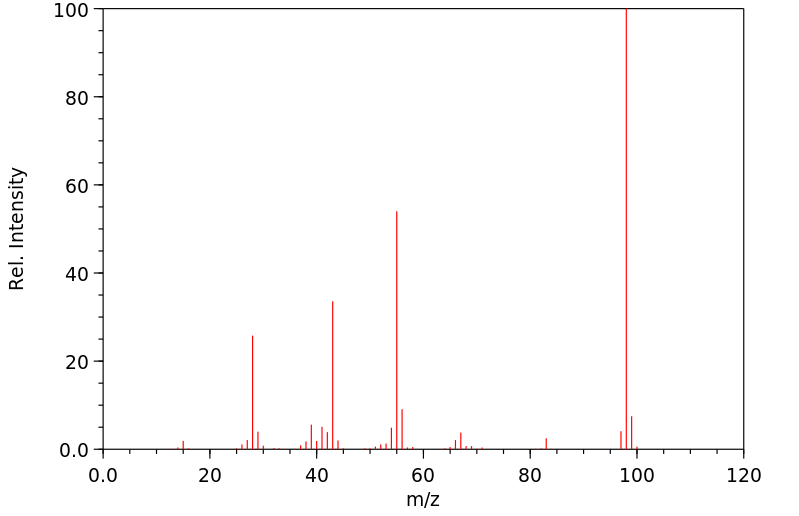
质谱MS
-
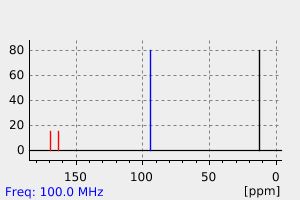
碳谱13CNMR
-
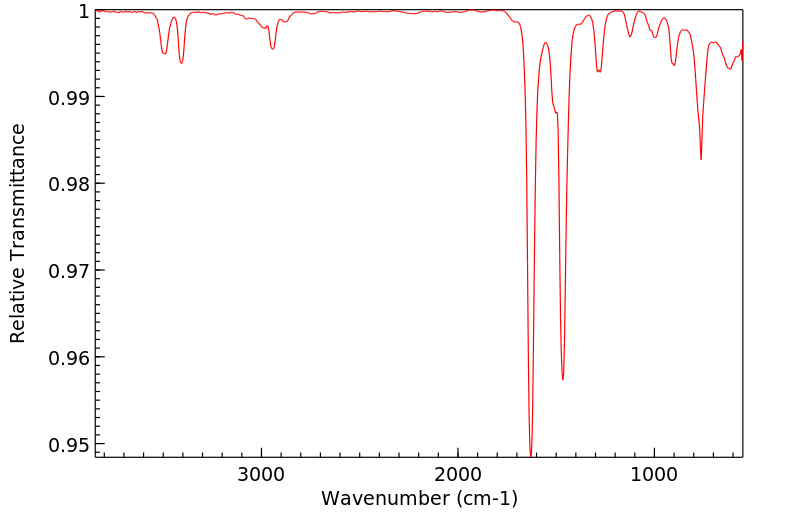
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
辛基羟乙基咪唑啉
辛基羟乙基咪唑啉
苯丙酸,3,4-二羟基-a-羰基-
肉豆蔻基羟乙基咪唑啉
硬脂酸,氨基乙基乙醇胺酰胺-咪唑啉,羧甲基化钠盐
甲基-(1-甲基-吡咯烷-2-亚基)-胺
甲基(5-甲基-1,2-恶唑-3-基)氨基甲酸酯
油基胺乙基咪唑啉
油基羟乙基咪唑啉
氯代醋酸钠与4,5-二氢-十一烷基-1H-咪唑-1-乙醇和氢氧化钠的反应产物
氯二甲基(1-甲基-1-丙烯基)硅烷
氯-乙酸反应产物与2-庚基-4,5-二氢-1H-咪唑-1-乙醇和氢氧化钠
月桂基羟乙基咪唑啉
恶唑-4-基氨基甲酸叔丁酯
异硬脂基羟乙基咪唑啉
异噁隆
异丙基亚氨基吡咯烷
噻唑-2,4-二胺
噁唑-4-胺
叔-丁基2-氨基-6,7-二氢吡唑并[1,5-A]吡嗪-5(4H)-甲酸基酯
十七碳-2-烯基-4,5-二氢-1H-咪唑-1-乙醇盐酸盐
偶氮引发剂VA-064
依凡达明
二氨基吡唑
乙基3-(乙基氨基)-5-甲基-1,2-恶唑-4-羧酸酯
alpha-(氯甲基)-2-异丙基-5-硝基-2H-咪唑-2-乙醇
alpha,4,4-三甲基-2-十一烷基-2-咪唑啉-1-乙醇
Z-2-(8-十七烯基)-4,5-二氢-1H-咪唑-1-乙醇
N-甲基异噻唑-3-胺盐酸
N-甲基-N-(5-甲基-3-异恶唑基)-乙酰胺
N-甲基-3-氨基吡唑
N-甲基-2-吡咯烷酮肟
N-甲基-1,2-噻唑-3-胺1,1-二氧化物
N-环己基-1,2-噻唑-3-胺1,1-二氧化物
N-叔-丁基-5-甲基-2H-吡唑-3-胺
N-乙基-N-(5-甲基-3-异恶唑基)-乙酰胺
N-乙基-1,2-噻唑-3-胺1,1-二氧化物
N-乙基-1,2,5-恶二唑-3,4-二胺
N-{(E)-[(4-氨基-1,2,5-恶二唑-3-基)氨基]亚甲基}乙酰胺
N-[2-[2-[(E)-十七碳-8-烯基]-4,5-二氢咪唑-1-基]乙基]乙烷-1,2-二胺
N-[2-[2-(13-二十一碳烯-1-基)-4,5-二氢-1H-咪唑-1-基]乙基]乙二胺
N-[2-(4,5-二氢-2-十九烷基-1H-咪唑-1-基)乙基]乙二胺
N-[2-(4,5-二氢-2-十一烷基-1H-咪唑-1-基)乙基]乙二胺
N-[(2Z)-哌嗪-2-亚基]-2,2,2-三氟乙酰肼
N-[(2-甲基苯基)氨基甲硫杂酰]-2-(4-羰基-2-苯基喹唑啉-3(4H)-基)-3-苯基丙酰胺
N-BOC-4-氨基噻唑
N-3-异恶唑氨基甲酸叔丁酯
N-(噻二唑-4-基)氨基甲酸乙酯
N-(5-叔丁基-1H-吡唑-3-基)氨基甲酸甲酯
N-(4,5-二甲基-3-异噁唑)氨基甲酸1,1-二甲基乙酯