2-((2-hydroxyethyl)amino)naphthalene-1,4-dione | 21102-27-2
中文名称
——
中文别名
——
英文名称
2-((2-hydroxyethyl)amino)naphthalene-1,4-dione
英文别名
2-(2-hydroxyethylamino)-1,4-naphthoquinone;2-(2-hydroxyethylamino)naphthalene-1,4-dione
CAS
21102-27-2
化学式
C12H11NO3
mdl
——
分子量
217.224
InChiKey
HDOTXWTUYIFUQH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:159.5-160.2 °C
-
沸点:423.3±45.0 °C(Predicted)
-
密度:1.34±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:66.4
-
氢给体数:2
-
氢受体数:4
SDS
上下游信息
反应信息
-
作为反应物:描述:2-((2-hydroxyethyl)amino)naphthalene-1,4-dione 在 copper(l) iodide 、 1,10-菲罗啉 、 morpholine-iodine complex 、 potassium carbonate 、 caesium carbonate 作用下, 以 二氯甲烷 、 水 、 甲苯 为溶剂, 反应 10.83h, 生成 3,4-dihydro-2H-naphtho[2,3-b][1,4]oxazine-5,10-dione参考文献:名称:具有生物活性的3,4-二氢-2 H-萘[2,3- b ] [1,4]恶嗪-5,10-二酮和2,3,4,5-四氢-1 H-萘[2,铜催化的分子内偶联反应生成3- b ]氮杂6,11-二酮衍生物摘要:3,4-二氢-2 H-萘[2,3- b ] [1,4]恶嗪-5,10-二酮和2,3,4,5-四氢-1 H-萘[2 ]的简便合成通过铜催化的分子内C-O / C-C偶联反应得到了3-,3 - b ]氮杂-6,11-二酮。该方法对官能团显示出良好的耐受性,并被用于天然产物核心结构的合成。一些偶联产物表现出对肺癌A549细胞的中等活性。DOI:10.1021/acs.joc.8b00020
-
作为产物:参考文献:名称:潜在生物还原烷基化醌的电化学。第4部分。叠氮基醌的定性和定量构效关系摘要:已经研究了生物还原烷基化作为叠氮基喹啉类抗癌剂作用机理的概念。研究了醌取代基对醌还原的影响,对醌还原前后氮丙啶的质子化的影响以及对化合物分配特性的影响。在描述这些过程的电化学,化学稳定性和亲脂性研究中获得的参数已确定,并在Hansch型QSAR研究中与从三个实验肿瘤模型获得的生物学数据进行了定量关联。在L 1210克隆形成试验中,细胞毒性与参数之间的定量相关性差。然而,体内抗肿瘤活性之间存在良好的线性关系(相对于L 1210)白血病小鼠和VS。发现B 16荷黑素瘤小鼠)和醌的亲脂性。这些关系显示出抗肿瘤活性和亲脂性之间的负相关关系,可以用来预测新的未知化合物的活性。尽管发现了一些关于取代基的电子和空间性质及其形成氢键能力的潜在重要性的迹象,但抗肿瘤活性与其他参数之间没有明显的趋势。DOI:10.1002/recl.19931120216
文献信息
-
Development of quinone analogues as dynamin GTPase inhibitors作者:Kylie A. MacGregor、Mohammed K. Abdel-Hamid、Luke R. Odell、Ngoc Chau、Ainslie Whiting、Phillip J. Robinson、Adam McCluskeyDOI:10.1016/j.ejmech.2014.06.070日期:2014.10Virtual screening of the ChemDiversity and ChemBridge compound databases against dynamin I (dynI) GTPase activity identified 2,5-bis-(benzylamino)-1,4-benzoquinone 1 as a 273 ± 106 μM inhibitor. In silico lead optimization and focused library-led synthesis resulted in the development of four discrete benzoquinone/naphthoquinone based compound libraries comprising 54 compounds in total. Sixteen analogues针对dynamin I(dynI)GTPase活性对ChemDiversity和ChemBridge化合物数据库进行的虚拟筛选确定了2,5-双-(苄氨基)-1,4-苯醌1为273±106μM抑制剂。在计算机上进行了铅优化和以库为主导的合成,开发了四个基于苯醌/萘醌的化合物库,总共包含54种化合物。16种类似物比铅1更有效,其中2,5-双-(4-羟基苯胺基)-1,4-苯醌(45)和2,5-双(4-羧基苯胺基)-1,4-苯醌(49) IC 50最活跃值分别为11.1±3.6和10.6±1.6μM。分子建模表明,dynI GTP结合位点内49个分子的稳定化涉及许多氢键和疏水相互作用。评价了六个活性最高的抑制剂对网格蛋白介导的内吞作用(CME)的潜在抑制作用。醌45是最有效的CME抑制剂,IC 50(CME)为36±16μM。
-
New Strategies for Molecular Diversification of 2-[Aminoalkyl-(1H-1,2,3-triazol-1- yl)]-1,4-naphthoquinones Using Click Chemistry作者:Ronaldo de Oliveira、Mauro da Silva、Moara da Silva、Valentina Melo、Wagner Valença、Josinete da Paz、Celso CamaraDOI:10.21577/0103-5053.20160207日期:——3-triazol-1-yl)ethylamino)]-3-(3-methylpropenyl)-1,4-naphthoquinones from lawsone with an overall yield of approximately 27% in six steps. On the other hand, convergent synthesis (Strategy B) was employed for the synthesis of 2-[(4-phenyl-1H-1,2,3-triazol-1-yl)alkyl-amino)]-3-(3-methylbut-2-en-1-yl)-1,4-naphthoquinones from the reaction between 2-methoxy-lapachol with amino-triazoles with a global yield of about基于点击化学的2-氨基-烷基-1,2,3-三唑-1,4-萘醌衍生物合成策略可从1,4-萘醌(1,4-NQ)获得所需的产物,以及生物基的法拉索,去甲拉帕胆和拉帕胆 从1,4-NQ和氨基醇开始的第一条路线(策略A),然后是2-氨基-烷基-1,4-NQ醇,被甲苯磺酸化。叠氮化物离子取代了甲苯磺酸酯基团,得到2-叠氮化物-烷基-1,4-NQ,将其置于铜催化的叠氮化物炔烃环加成(CuAAC)条件下。以大约47%的总产率获得了三唑-萘醌。另一种途径(策略B)使用NaN3作为亲核试剂,依次进行CuAAC取代邻苯二甲酰亚胺,并用肼生成氨基三唑对邻苯二甲酰亚胺基团进行脱保护。随后的反应是,1 4-NQ分四步生产了2-氨基-烷基-1,2,3-三唑-1,4-NQ衍生物,总产率为45-76%。在我们开发了这两种策略之后,选择了线性合成方法(策略A)来制备2-[((2-(1H-1,2,3-三唑-1-基)乙基氨基)]
-
Bifunctionalisation of 1,4-naphthoquinone by the Oxidative Addition of an Alkylamine and Iodine作者:Huan-Ming Huang、Yu-Jin Li、Yin-Ping Dai、Wu-Bin Yu、Qin Ye、Jian-Rong GaoDOI:10.3184/174751912x13547276507240日期:2013.1
Novel 2-iodo-3-(alkylamino) naphthalene-1,4-diones are formed in 33–70% yield by the reaction of alkylamine and 1, 4-naphthoquinone in the presence of iodine.
-
[EN] SMALL MOLECULE NAPHTHOQUINONE- AND PHTHALIMIDE-BASED LIPOCATIONS AS ANTI-PARASITIC AGENTS<br/>[FR] LIPOCATIONS À BASE DE NAPHTOQUINONE ET DE PHTALIMIDE À PETITES MOLÉCULES EN TANT QU'AGENTS ANTI-PARASITAIRES申请人:UNIV GEORGIA公开号:WO2013036766A1公开(公告)日:2013-03-14Small molecule naphthoquinone- and phthalimide-based lipocations are provided, as well as methods for their use in treating or preventing anti-parasitic diseases, such as malaria, Chagas disease, and African Sleeping Sickness.
-
Synthetic enamine naphthoquinone derived from lawsone as cytotoxic agents assessed by in vitro and in silico evaluations作者:Bárbara C. Lemos、Regina Westphal、Eclair Venturini Filho、Rodolfo G. Fiorot、José Walkimar M. Carneiro、Anne Caroline C. Gomes、Celina J. Guimarães、Fátima C.E. de Oliveira、Pedro Mikael S. Costa、Claudia Pessoa、Sandro J. GrecoDOI:10.1016/j.bmcl.2021.128419日期:2021.12We synthesized ten enamine naphthoquinones with yields ranging from 43 to 76%. These compounds were screened for their in vitro antiproliferative activities by MTT assay against four types of human cancer cell lines: HCT116, PC3, HL60 and SNB19. The naphthoquinones bearing the picolylamine (7) and quinoline (12) moieties were the most actives (IC50 < 24 μM for all the cell lines), which were comparable
表征谱图
-
氢谱1HNMR
-
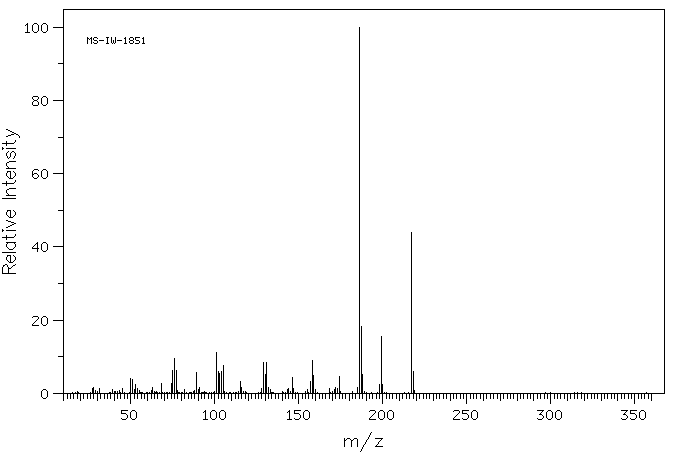
质谱MS
-
碳谱13CNMR
-
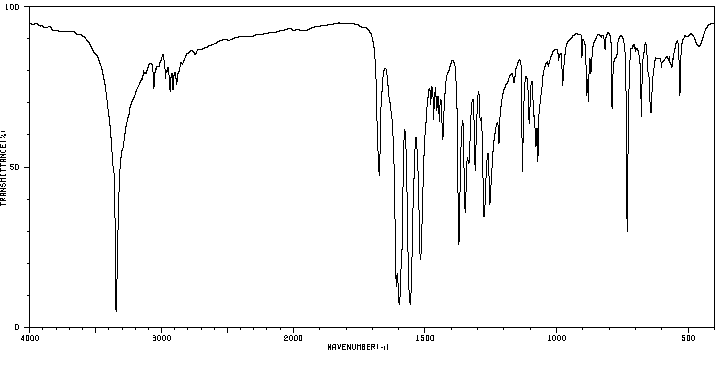
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮