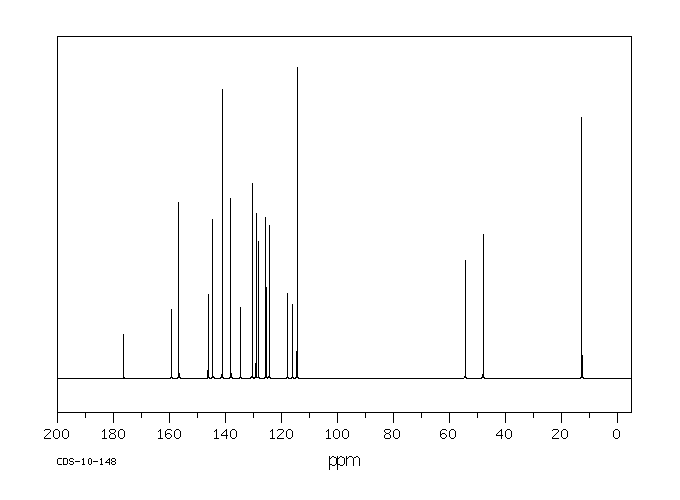
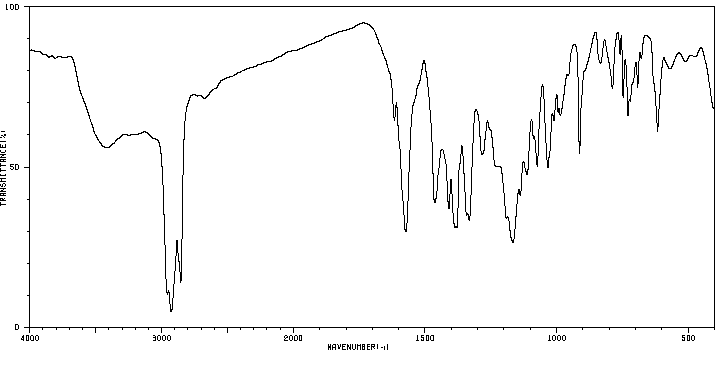
毒理性
国际癌症研究机构致癌物:快速绿 FCF
IARC Carcinogenic Agent:Fast Green FCF
来源:International Agency for Research on Cancer (IARC)