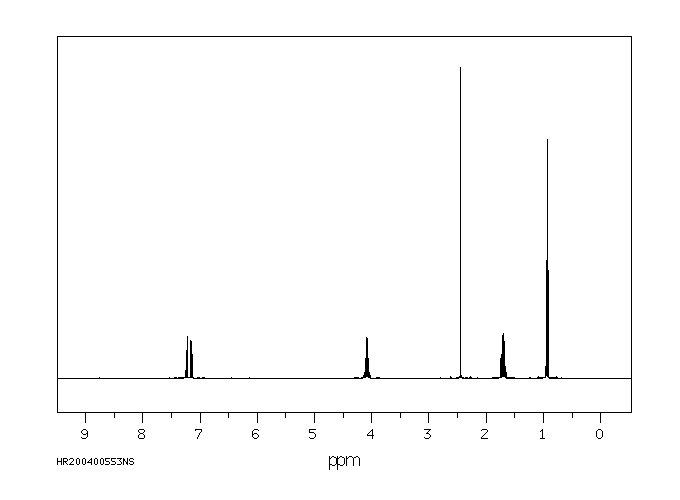
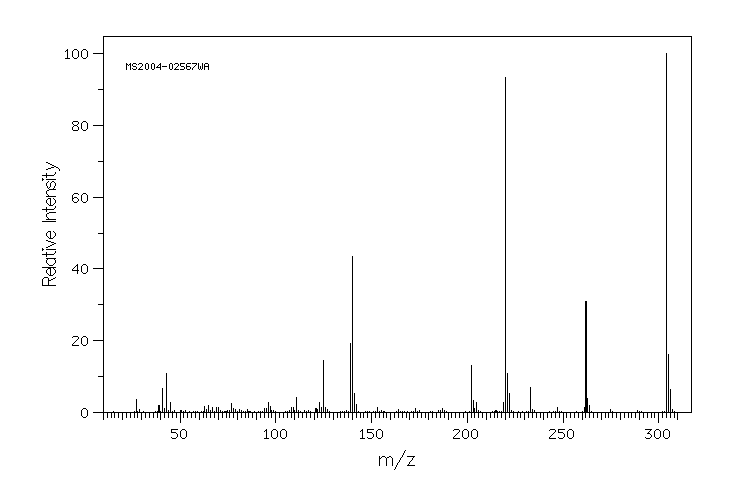
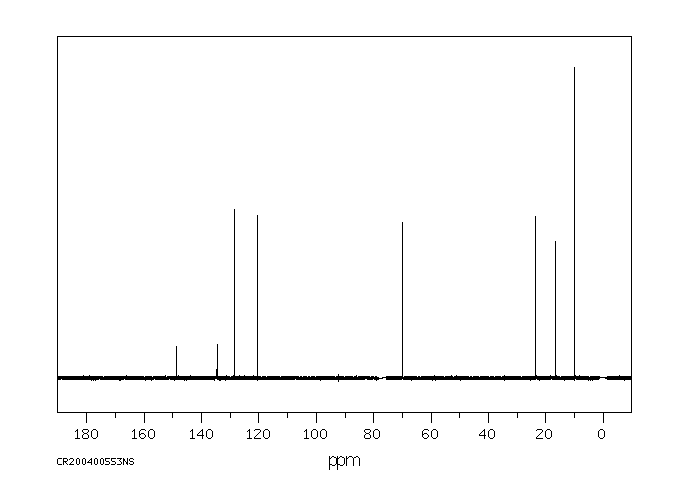
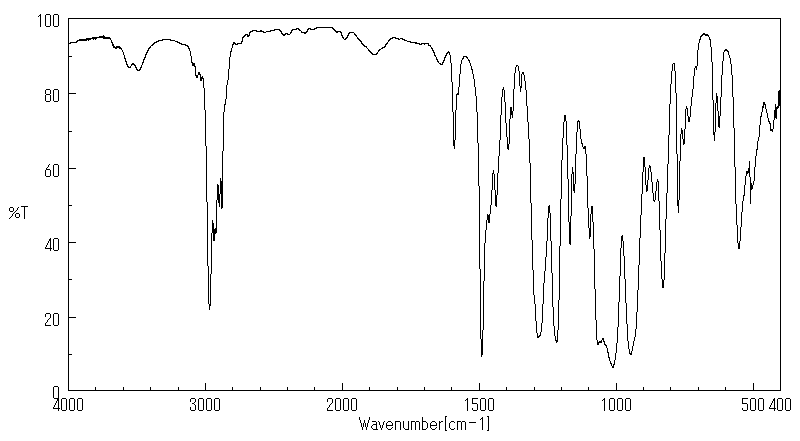
代谢
众所周知,大多数有机磷杀虫剂的活性代谢物在一定程度上会与组织酯酶(除AChE外)发生共价反应。由于这些酯酶对健康的重要性不大,因此这种结合反应可以被视为一种解毒过程。尽管这些酯酶的催化活性很高,但这类位点的实际数量相对较少……主要结合部位在肝脏和肌肉。结合的量不会因为施用的有机磷杀虫剂的LD50而有很大差异。因此,只有对于像对氧磷这样非常有毒的化合物,结合的量才占总剂量的一个重要比例,而对于LD50更高的化合物则不然。然而,在某些情况下,结合位点的数量与逃避其他代谢清除过程的循环抗胆碱酯酶氧醛的数量相比,可能是非常显著的。绑定到这些非关键位点的氧醛分子被阻止攻击AChE或NTE等关键位点。因此,结合位点可以被认为是防止中毒的重要第二道防线。/有机磷农药/
It is well-known that active metabolites of most organophosphorus insecticides react covalently to some extent with tissue esterases other than AChE. Since few of these esterases appear vital to health, the binding reaction may be considered a detoxification process. Although the catalytic activity of these esterases is high, the actual quantity of such sites is comparatively small. ... Binding was principally in liver and muscle. The quantity bound would not be expected to be much greater whatever the LD50 of an administered organophosphorus insecticide. Thus, it is a significant proportion of the total dose only for a very toxic compound such as paraoxon but not for compounds with much higher LD50s. However, the number of binding sites may, in some cases, be very significant compared with the quantity of circulating anticholinesterase oxon that has avoided other metabolic disposal processes. Molecules of oxon bound to these non-vital sites are prevented from attacking the vital sites such as AChE or NTE. Binding sites can therefore be considered an important second line of defense against intoxication. /Organophosphorus pesticides/
来源:Hazardous Substances Data Bank (HSDB)