代谢
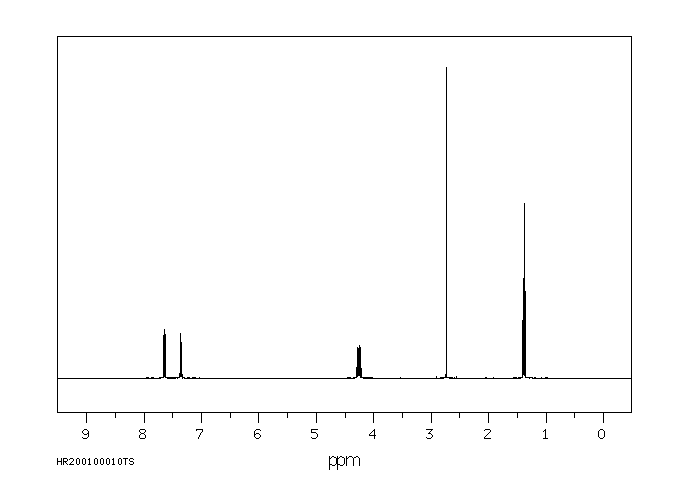
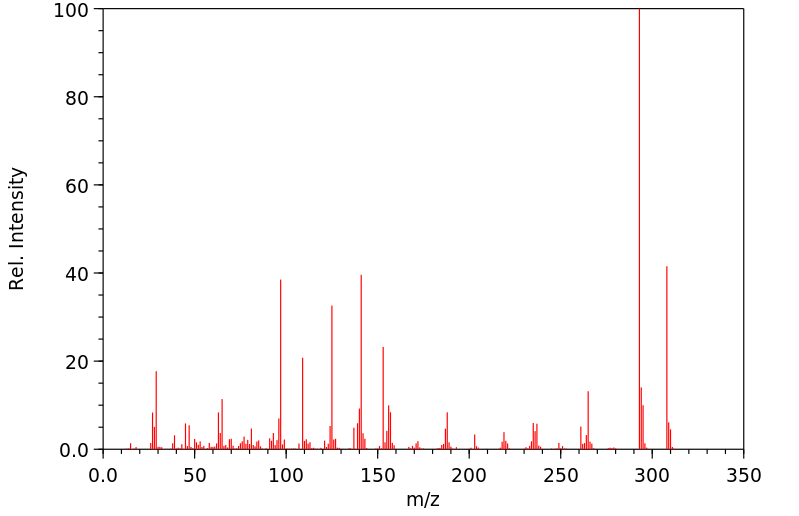
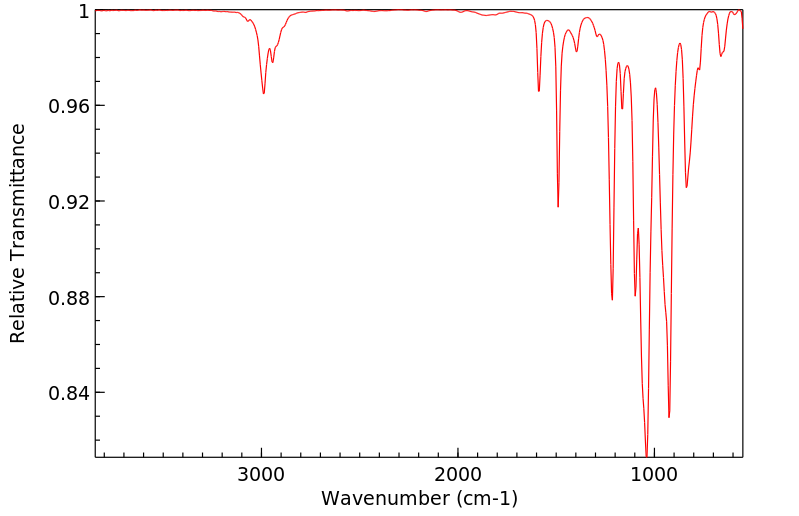
(32)P标记的达沙尼特应用于幼棉植株的根和茎部...在茎部应用的情况下,超过80%的放射性是以母体化合物的形式存在。主要代谢物是氧类似物和达沙尼特磺酰。9天后,还检测到了少量的氧类似物磺酰。...通过对蟑螂进行代谢研究,表明存在达沙尼特的异构体和磺酰的S-乙基异构体。
(32)P-LABELED DASANIT WAS APPLIED TO /ROOTS &/ STEMS OF YOUNG COTTON PLANTS ... IN THE CASE OF STEM APPLICATION, MORE THAN 80% OF THE RADIOACTIVITY WAS IN THE FORM OF THE PARENT CMPD. THE MAJOR METABOLITES WERE THE OXYGEN ANALOGUE & DASANIT SULFONE. AFTER 9 DAYS, SMALL AMT OF THE OXYGEN ANALOGUE SULFONE WAS ALSO DETECTED. ... METABOLISM STUDIES WITH COCKROACHES HAVE INDICATED PRESENCE OF THE ISOMERIDE & SULFONE OF DASANIT & THE S-ETHYL ISOMER OF THE SULFONE.
来源:Hazardous Substances Data Bank (HSDB)