乙酰硫代氨基脲 | 2302-88-7
中文名称
乙酰硫代氨基脲
中文别名
1-乙酰-3-硫氨基脲;4-乙酰基氨基硫脲
英文名称
1-acetylthiosemicarbazide
英文别名
2-acetylhydrazinecarbothioamide;1-Acetyl-thiosemicarbazid;acetylthiosemicarbazide;1-acetyl-3-thiosemicarbazide;N-(carbamothioylamino)acetamide;Acetyl-3-thiosemicarbazid;acetamidothiourea
CAS
2302-88-7
化学式
C3H7N3OS
mdl
MFCD06682722
分子量
133.174
InChiKey
NSIMQTOXNOFWBP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:165-170 °C
-
密度:1.280 (estimate)
-
溶解度:DMSO(少量)、甲醇(少量)
-
稳定性/保质期:
在常温常压下稳定,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):-0.9
-
重原子数:8
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:99.2
-
氢给体数:3
-
氢受体数:2
安全信息
-
危险等级:6.1
-
危险品标志:T
-
安全说明:S24/25
-
危险类别码:R36/37/38,R25
-
海关编码:2930909090
-
危险品运输编号:2811
-
包装等级:III
-
危险类别:6.1
-
危险性防范说明:P264,P270,P301+P310,P321,P330,P405,P501
-
危险性描述:H301
SDS
1.1 产品标识符
: 1-乙酰-3-硫氨基脲
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H301 吞咽会中毒
警告申明
预防
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
措施
P301 + P310 如果吞下去了: 立即呼救解毒中心或医生。
P321 具体治疗(见本标签上提供的急救指导)。
P330 漱口。
储存
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C3H7N3OS
分子式
: 133.17 g/mol
分子量
组分 浓度或浓度范围
1-(Acetyl)thiOSemicarbazide
-
CAS 号 2302-88-7
EC-编号 218-956-4
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 立即将患者送往医院。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
无数据资料
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
戴呼吸罩。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 将人员撤离到安全区域。
避免吸入粉尘。
6.2 环境保护措施
在确保安全的前提下,采取措施防止进一步的泄漏或溢出。 不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
避免与皮肤、眼睛和衣服接触。 休息以前和操作过此产品之后立即洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能微粒防毒面具N99型(US)
或P2型(EN
143)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防毒
面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 165 - 168 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
4.6 - (空气= 1。0)
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
辛醇--水的分配系数的对数值: -1.138
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 误吞会中毒。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 2811 国际海运危规: 2811 国际空运危规: 2811
14.2 联合国(UN)规定的名称
欧洲陆运危规: TOXIC SOLID, ORGANIC, N.O.S. (1-(Acetyl)thiOSemicarbazide)
国际海运危规: TOXIC SOLID, ORGANIC, N.O.S. (1-(Acetyl)thiOSemicarbazide)
国际空运危规: Toxic solid, organic, n.o.s. (1-(Acetyl)thiOSemicarbazide)
14.3 运输危险类别
欧洲陆运危规: 6.1 国际海运危规: 6.1 国际空运危规: 6.1
14.4 包裹组
欧洲陆运危规: III 国际海运危规: III 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 1-乙酰-3-硫氨基脲
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H301 吞咽会中毒
警告申明
预防
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
措施
P301 + P310 如果吞下去了: 立即呼救解毒中心或医生。
P321 具体治疗(见本标签上提供的急救指导)。
P330 漱口。
储存
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C3H7N3OS
分子式
: 133.17 g/mol
分子量
组分 浓度或浓度范围
1-(Acetyl)thiOSemicarbazide
-
CAS 号 2302-88-7
EC-编号 218-956-4
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 立即将患者送往医院。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
无数据资料
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
戴呼吸罩。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 将人员撤离到安全区域。
避免吸入粉尘。
6.2 环境保护措施
在确保安全的前提下,采取措施防止进一步的泄漏或溢出。 不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
避免与皮肤、眼睛和衣服接触。 休息以前和操作过此产品之后立即洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能微粒防毒面具N99型(US)
或P2型(EN
143)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防毒
面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 165 - 168 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
4.6 - (空气= 1。0)
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
辛醇--水的分配系数的对数值: -1.138
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 误吞会中毒。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 2811 国际海运危规: 2811 国际空运危规: 2811
14.2 联合国(UN)规定的名称
欧洲陆运危规: TOXIC SOLID, ORGANIC, N.O.S. (1-(Acetyl)thiOSemicarbazide)
国际海运危规: TOXIC SOLID, ORGANIC, N.O.S. (1-(Acetyl)thiOSemicarbazide)
国际空运危规: Toxic solid, organic, n.o.s. (1-(Acetyl)thiOSemicarbazide)
14.3 运输危险类别
欧洲陆运危规: 6.1 国际海运危规: 6.1 国际空运危规: 6.1
14.4 包裹组
欧洲陆运危规: III 国际海运危规: III 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Antitumor activity of 5-substituted 2-acylamino-1,3,4-thiadiazoles against transplantable rodent tumors.摘要:## clewd修改版 v4.8(11) by tera **claude-3-5-sonnet-20241022 error**: 404 ```Unknown chat conversation 7306a1e6-ee53-4b68-88ad-42503b2221d9``` FAQ: https://rentry.org/teralomaniac_clewdDOI:10.1248/cpb.33.5126
-
作为产物:参考文献:名称:1,2,4-三唑-3-硫酮化合物作为二嗪金属β-内酰胺酶的抑制剂。摘要:金属β-内酰胺酶(MBLs)引起革兰氏阴性细菌对β-内酰胺类抗生素的耐药性并引起人们的严重关注,因为它们可以使最后的碳青霉烯类药物失活,并且仍然缺乏具有临床价值的MBL抑制剂。我们之前确定了在其二嗪活性位点内4-氨基-2,4-二氢-5-(2-甲基苯基)-3H-1,2,4-三唑-3-硫酮(化合物IIIA)的原始结合模式。 L1 MBL。在这里,我们提出了L1与相应的非氨基化合物IIIB(1,2-二氢-5-(2-甲基苯基)-3H-1,2,4-三唑-3-硫酮)的配合物的晶体结构。出乎意料的是,IIIB的结合模式与IIIA相似,但相反。3D结构表明三唑-硫酮骨架适合结合二锌金属酶的催化位点。根据这些结果,我们合成了IIIA或IIIB的54个类似物。有19个显示的IC50值在微摩尔范围内,朝着五个代表性MBL(即L1,VIM-4,VIM-2,NDM-1和IMP-1)中的至少一个显示。其中五个对至少四DOI:10.1002/cmdc.201700186
-
作为试剂:描述:stannous lactate 在 乙酰硫代氨基脲 作用下, 110.0~180.0 ℃ 、133.32 Pa 条件下, 反应 2.0h, 以95.82%的产率得到(3R,6R)-3,6-二甲基-1,4-二恶烷-2,5-二酮参考文献:名称:一种催化合成丙交酯的方法摘要:本发明公开了一种催化合成丙交酯的方法,乳酸亚锡与脲类物质的混合物为复合催化剂,以乳酸含量90%的L‑乳酸(或D‑乳酸)为原料,采用减压蒸馏技术合成L‑丙交酯(或D‑丙交酯),相较于单独使用其中一种催化剂而言,采用复合催化剂可以实现产率有效提升,在相同实验条件下,单独使用乳酸亚锡或脲类催化剂合成丙交酯的粗产率分别为69%‑72%和23%‑30%,而两者复合催化剂可提升至90%以上。相较于传统锡类催化剂或锌类催化剂以及其他复合催化组分而言,本发明复合催化反应体系的反应温度低(150‑180℃),反应时间短(0.5‑2h),丙交酯产率高(90%以上),更加节能增产,有利于工业化生产。公开号:CN112250661A
文献信息
-
Synthesis of 1-Chloroacetyl-1-dehydroxy-2,3,5-tri-O-benzoyl-β-D-ribofuranose. A Potentially Versatile Intermediate for the Synthesis of C-Nucleosides作者:Hyo Kyung Han、Jae Chul Lee、Yong Han Kang、Joong-Hyup Kim、Dae Yoon ChiDOI:10.1080/00397919208021547日期:1992.11Abstract New key carbohydrate intermediate, 1-chloroacetyl-1-dehydroxy-2,3,5-tri-O-benzoyl-β-D-ribofuranose (1a), has been synthesized. The application of this key intermediate would be very wide. We have very conveniently synthesized several C-nucleosides including monocyclic and 5,5-membered bicyclic compounds from this intermediate.
-
Pyrazole-Based Lactate Dehydrogenase Inhibitors with Optimized Cell Activity and Pharmacokinetic Properties作者:Ganesha Rai、Daniel J. Urban、Bryan T. Mott、Xin Hu、Shyh-Ming Yang、Gloria A. Benavides、Michelle S. Johnson、Giuseppe L. Squadrito、Kyle R. Brimacombe、Tobie D. Lee、Dorian M. Cheff、Hu Zhu、Mark J. Henderson、Katherine Pohida、Gary A. Sulikowski、David M. Dranow、Md Kabir、Pranav Shah、Elias Padilha、Dingyin Tao、Yuhong Fang、Plamen P. Christov、Kwangho Kim、Somnath Jana、Pavan Muttil、Tamara Anderson、Nitesh K. Kunda、Helen J. Hathaway、Donna F. Kusewitt、Nobu Oshima、Murali Cherukuri、Douglas R. Davies、Jeffrey P. Norenberg、Larry A. Sklar、William J. Moore、Chi V. Dang、Gordon M. Stott、Leonard Neckers、Andrew J. Flint、Victor M. Darley-Usmar、Anton Simeonov、Alex G. Waterson、Ajit Jadhav、Matthew D. Hall、David J. MaloneyDOI:10.1021/acs.jmedchem.0c00916日期:2020.10.8of compounds, using structure-based design concepts, coupled with optimization of cellular potency, in vitro drug–target residence times, and in vivo PK properties, to identify first-in-class inhibitors that demonstrate LDH inhibition in vivo. The lead compounds, named NCATS-SM1440 (43) and NCATS-SM1441 (52), possess desirable attributes for further studying the effect of in vivo LDH inhibition.乳酸脱氢酶(LDH)催化丙酮酸向乳酸的转化,同时还原的烟酰胺腺嘌呤二核苷酸的氧化是糖酵解途径的最后一步。糖酵解在癌细胞的代谢可塑性中起重要作用,长期以来一直被认为是潜在的治疗靶点。因此,LDH的有效的选择性抑制剂代表了一种有吸引力的治疗方法。然而,迄今为止,药理剂未能实现体内显着的靶标结合,可能是因为蛋白质以非常高的浓度存在于细胞中。我们在此报告了一项领先的优化运动,该研究使用基于结构的设计概念,重点研究了吡唑基系列化合物,并在体外优化了细胞效能药物-靶标的停留时间和体内PK特性,以鉴定可证明LDH在体内具有抑制作用的一流抑制剂。名为NCATS-SM1440(43)和NCATS-SM1441(52)的先导化合物具有进一步研究体内LDH抑制作用所需的属性。
-
Synthesis of New Surfactants Mono and Bipolar Derived from 1,2,4‐Triazole‐5‐thione作者:Driss Chebabe、Ahmed Dermaj、Zine El Abidine Ait Chikh、Najat Hajjaji、Isabelle Rico‐Lattes、Armand LattesDOI:10.1081/scc-200036625日期:2004.12.31first is a condensation of the thiosemicarbazide with the benzoyl chloride and the acetyl chloride for the PTS and MTS respectively in pyridinic medium. This step leads to the formation of 1‐benzoylthiosemicarbazide and 1‐acetylthiosemicarbazide. The 1‐acetylthiosemicarbazide is also prepared with a new method consisted of a simple solvolysis the thiosemicarbazide in acetic acid for 4 hr. The second is
-
The synthesis, X-ray crystal structure and optical properties of novel 1-ferrocenyl-2-(3-phenyl-1H-1,2,4-triazol-5-ylthio)ethanone derivatives作者:Wei-Yong Liu、Yong-Sheng Xie、Bao-Xiang Zhao、Song Lian、Hong-Shui Lv、Zhong-Liang Gong、Dong-Soo ShinDOI:10.1016/j.saa.2010.04.019日期:2010.91-ferrocenyl-2-(3-phenyl-1H-1,2,4-triazol-5-ylthio)ethanone derivatives was synthesized by the reaction of 3-substituted-1H-1,2,4-triazole-5-thiol and chloroacetyl ferrocene in the presence of sodium hydride and potassium iodide at reflux. The structures of the new compounds were determined by IR and 1H NMR spectroscopy and HRMS. The structure of compound 5c was established by X-ray crystallography. UV–vis
-
Discovery and Optimization of Potent, Cell-Active Pyrazole-Based Inhibitors of Lactate Dehydrogenase (LDH)作者:Ganesha Rai、Kyle R. Brimacombe、Bryan T. Mott、Daniel J. Urban、Xin Hu、Shyh-Ming Yang、Tobie D. Lee、Dorian M. Cheff、Jennifer Kouznetsova、Gloria A. Benavides、Katie Pohida、Eric J. Kuenstner、Diane K. Luci、Christine M. Lukacs、Douglas R. Davies、David M. Dranow、Hu Zhu、Gary Sulikowski、William J. Moore、Gordon M. Stott、Andrew J. Flint、Matthew D. Hall、Victor M. Darley-Usmar、Leonard M. Neckers、Chi V. Dang、Alex G. Waterson、Anton Simeonov、Ajit Jadhav、David J. MaloneyDOI:10.1021/acs.jmedchem.7b00941日期:2017.11.22pyrazole-based inhibitors of human lactate dehydrogenase (LDH). Utilization of a quantitative high-throughput screening paradigm facilitated hit identification, while structure-based design and multiparameter optimization enabled the development of compounds with potent enzymatic and cell-based inhibition of LDH enzymatic activity. Lead compounds such as 63 exhibit low nM inhibition of both LDHA and我们报告了一系列新型吡唑类人乳酸脱氢酶 (LDH) 抑制剂的发现和药物化学优化。定量高通量筛选范例的利用促进了命中鉴定,而基于结构的设计和多参数优化使得能够开发出对 LDH 酶活性具有有效酶促和基于细胞抑制作用的化合物。 63等先导化合物在 MiaPaCa2 胰腺癌和 A673 肉瘤细胞中表现出对 LDHA 和 LDHB 的低 nM 抑制作用、对乳酸产生的亚微摩尔抑制作用以及对糖酵解的抑制作用。此外,使用细胞热位移测定 (CETSA) 证明了先导化合物对 LDHA 的强大靶向作用,并通过 SPR 确定了药物-靶标停留时间。对这些数据的分析表明,药物靶点停留时间(解离速率)可能是获得对该癌症代谢靶点的有效细胞抑制的一个重要因素。
表征谱图
-
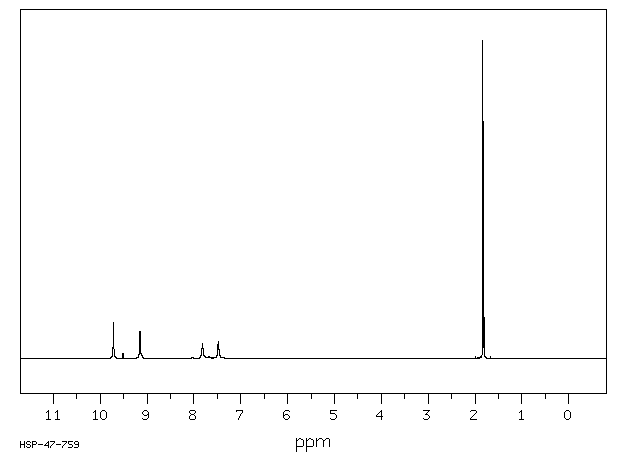
氢谱1HNMR
-
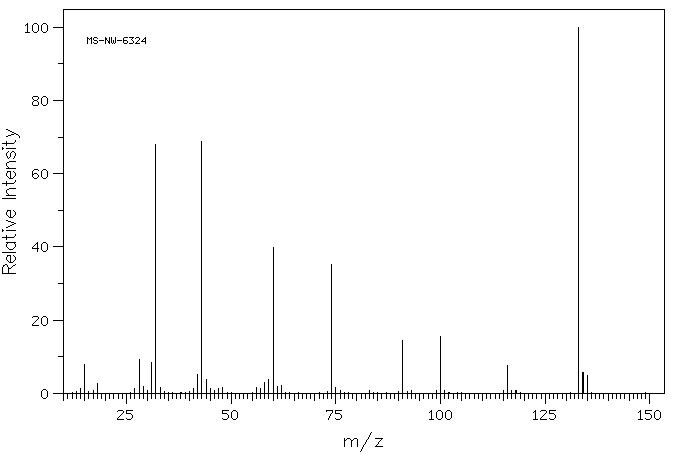
质谱MS
-
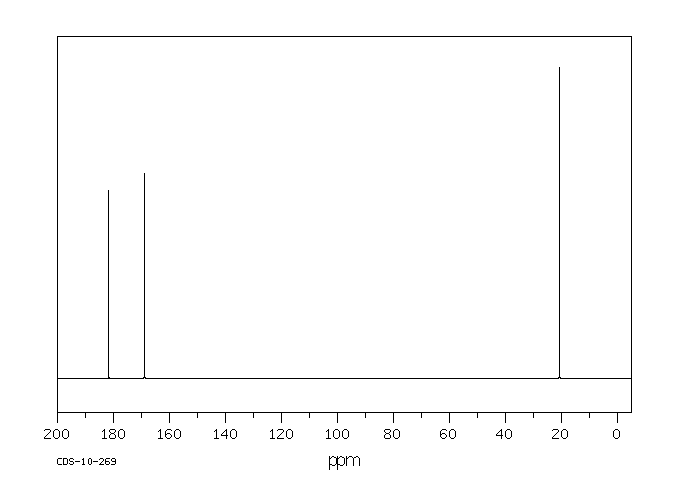
碳谱13CNMR
-
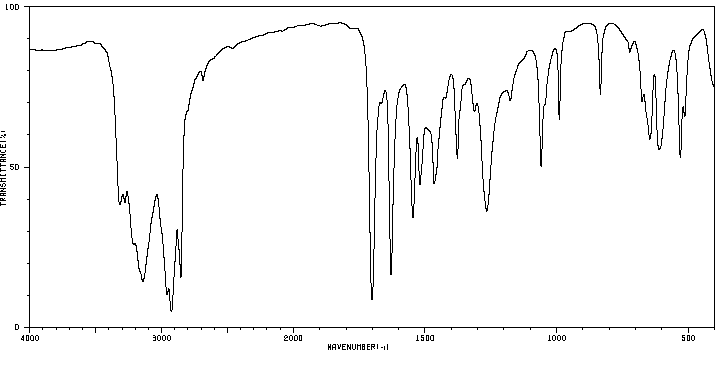
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷