二苯并[2,3,7,8]中氮茚 | 239-40-7
中文名称
二苯并[2,3,7,8]中氮茚
中文别名
——
英文名称
dibenzo<2,3;7,8>indolizine
英文别名
dibenz<2,3;7,8>indolizine;indolo<1,2-a>isoquinoline;indolo<2,1-a>isoquinoline;dibenzoindolizine;indolo[2,1-a]isoquinoline;indolo[2,1-a]isoquinoline
CAS
239-40-7
化学式
C16H11N
mdl
——
分子量
217.27
InChiKey
NHJPXGYCHHDLDD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):5.1
-
重原子数:17
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:4.4
-
氢给体数:0
-
氢受体数:0
反应信息
-
作为反应物:描述:二苯并[2,3,7,8]中氮茚 在 sodium tetrahydroborate 、 三氟乙酸 作用下, 以 四氢呋喃 为溶剂, 反应 6.0h, 以20%的产率得到5,6-dihydroindolo[2,1-a]isoquinoline参考文献:名称:摘要:Condensation of 5,6-dihydroindolo[2,1-a]isoquinoline with aromatic aldehydes in trifluoroacetic acid afforded 12-arylidene-5,6-dihydroindolo[2,1-a]isoquinolinium trifluoroacetates. Hydrogenolysis of these salts on rhenium heptasulfide at elevated temperature and hydrogen pressure yielded indolo[2,1-a]isoquinolines, while reduction with sodium borohydride gave 12-arylmethylindoloisoquinolines. Photoluminescence was found for some indolo[2,1-a]isoquinolines.DOI:10.1023/a:1021684515325
-
作为产物:参考文献:名称:Synthesis of 2,3,8,9-tetramethoxy-11-phenyl-5,6-dihydrodibenz[2,3;7,8]indolizine摘要:DOI:10.1007/bf00534392
文献信息
-
A Combined Experimental and Computational Study on the Cycloisomerization of 2-Ethynylbiaryls Catalyzed by Dicationic Arene Ruthenium Complexes作者:Yoshihiko Yamamoto、Kazuma Matsui、Masatoshi ShibuyaDOI:10.1002/chem.201500248日期:2015.5.4Ruthenium‐catalyzed cycloisomerization of 2‐ethynylbiaryls was investigated to identify an optimal ruthenium catalyst system. A combination of [η6‐(p‐cymene)RuCl2(PR3)] and two equivalents of AgPF6 effectively converted 2‐ethynylbiphenyls into phenanthrenes in chlorobenzene at 120 °C over 20 h. Moreover, 2‐ethynylheterobiaryls were found to be favorable substrates for this ruthenium catalysis, thus
-
A concise synthesis of indolo[2,1-a]isoquinoline via alkyne annulations promoted by base作者:Hina Mehmood、Muhammad Asif Iqbal、Ruimao HuaDOI:10.1016/j.tetlet.2021.153566日期:2022.1A convenient and one-pot synthetic method for the construction of indolo[2,1-a]isoquinolines is developed by the dehydrative cyclocondensation reactions of the easily available 2-hydroxymethyl anilines with 2-ethynylbenzaldehydes in DMSO in the presence of KOH and TBAI under air.
-
Synthesis of Phenanthrenes and Polycyclic Heteroarenes by Transition-Metal Catalyzed Cycloisomerization Reactions作者:Victor Mamane、Peter Hannen、Alois FürstnerDOI:10.1002/chem.200400220日期:2004.9.20Readily available biphenyl derivatives containing an alkyne unit at one of their ortho-positions are converted into substituted phenanthrenes on exposure to catalytic amounts of either PtCl2, AuCl, AuCl3, GaCl3 or InCl3 in toluene. This 6-endo-dig cyclization likely proceeds through initial pi-complexation of the alkyne unit followed by interception of the resulting eta2-metal species by the adjacent在暴露于催化量的甲苯中的PtCl2,AuCl,AuCl3,GaCl3或InCl3时,容易获得的在邻位之一含有炔单元的联苯衍生物会转化为取代的菲。这种6-endo-dig环化反应可能是通过炔烃单元的最初pi络合反应进行,然后被相邻的芳烃环截获所得的eta2-金属。该反应固有地是模块化的,从而允许实质性的结构变化并允许在菲产物的任何位点上引入取代基。此外,它容易扩展到杂环系列,例如制备苯并吲哚,苯并咔唑,萘噻吩以及桥头氮杂环如吡咯并[1,2-a]喹啉。根据选择的催化剂,带有卤代炔单元的联芳基可以转化为相应的10-卤菲或异构体的9-卤菲。在后一种情况下,最好以金属亚乙烯基作为反应性中间体来解释伴随的1,2-卤化物移位。通过一系列与抗癌药康布雷他汀A-4的近亲相接的多加氧菲的全合成,以及阿波啡碱生物碱O-的全合成,可以说明这种制备多环芳烃的新方法的范围。甲基-脱氢异piline及其天然对称的二聚体
-
Preventives and remedies for central nervous system diseases申请人:——公开号:US20030207863A1公开(公告)日:2003-11-06A prophylactic or therapeutic agent for central nervous system diseases based on amyloid &bgr;40 secretion inhibitory activity of a compound having urotensin II receptor antagonistic activity or a salt thereof.一种预防或治疗中枢神经系统疾病的药剂,基于一种具有尿泰松II受体拮抗活性或其盐的化合物对淀粉样蛋白β40分泌抑制活性。
-
Preventives/remedies for urinary disturbance申请人:Ishihara Yuji公开号:US20050197362A1公开(公告)日:2005-09-08Preventives/remedies for voiding disturbance containing a compound having both of an acetylcholinesterase inhibitory action and an α1 antagonistic action which exhibits an excellent effect of improving the urinary function of the bladder (i.e., effects of improving urine flow rate and voiding efficiency) without affecting the urinary pressure or the blood pressure.
表征谱图
-
氢谱1HNMR
-
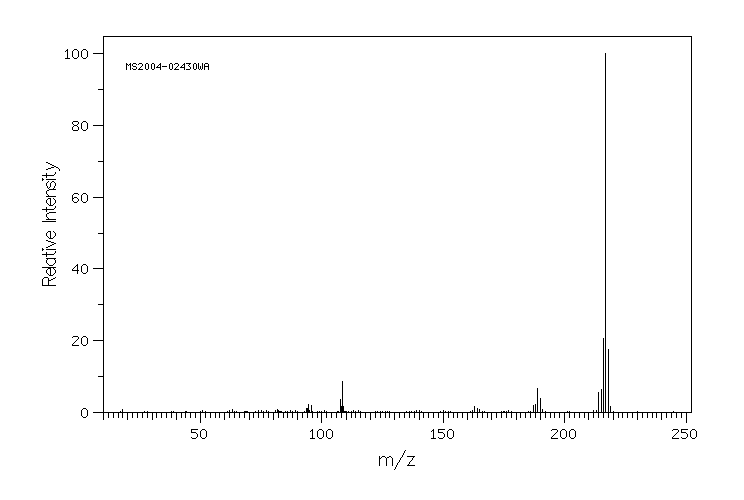
质谱MS
-
碳谱13CNMR
-
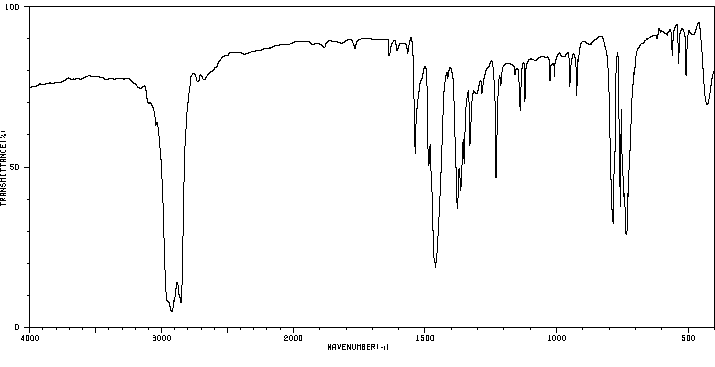
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(4-(4-氯苯基)硫代)-10-甲基-7H-benzimidazo(2,1-A)奔驰(德)isoquinolin-7一
高氯酸9-碘-11-甲基吡啶并[1,2-b]异喹啉正离子
高唐碱
顺阿曲库胺草酸盐
顺苯磺阿曲库铵叔丁酯异构体
降氧化北美黄连次碱
阿莫伦特
阿特拉库铵杂质20
阿特拉库铵杂质19
阿特拉库铵杂质19
阿曲库铵杂质V
阿曲库铵杂质N
阿曲库铵杂质J
阿曲库铵杂质I
阿曲库铵杂质F
阿曲库铵杂质E
阿曲库铵杂质E
阿曲库铵杂质D2
阿曲库铵杂质D
阿曲库铵杂质C
阿曲库铵杂质A
阿曲库铵杂质8
阿曲库铵杂质48
阿曲库铵杂质47
阿曲库铵杂质1
阿曲库铵EP杂质D
阿曲库铵
阿曲库胺草酸盐
阿司他丁
阿区库铵去甲基杂质
长茎唐松碱
过氧荧光素1
贝马力农
衡州乌药碱; 乌药碱
蝙蝠葛碱
蝙蝠葛新林碱
蒂巴因水杨酸盐
葡萄孢镰菌素
萘酞磷
萘氨磷
萘亚胺
莲心季铵碱
莲子心碱
莫沙维林
苯酚,4-[(1,2,3,4-四氢-2-甲基-1-异喹啉基)甲基]-
苯磺顺阿曲库铵杂质23
苯磺安托肌松
苯并咪唑并[2,1-A]苯并[D,E]异奎千酮-7-酮
苯并[g]异喹啉-5,10-二酮
苯并[f]异喹啉-4(3h)-酮