双(三甲基硅基甲基)硫醚 | 4712-51-0
中文名称
双(三甲基硅基甲基)硫醚
中文别名
双(三甲基硅甲基)硫醚;硫代二(亚甲基)二(三甲基硅烷)
英文名称
bis(trimethylsilylmethyl) sulfide
英文别名
trimethyl(trimethylsilylmethylsulfanylmethyl)silane
CAS
4712-51-0
化学式
C8H22SSi2
mdl
MFCD00039797
分子量
206.5
InChiKey
GISUCMFTNKRCPF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:88 °C/21 mmHg (lit.)
-
密度:0.844 g/mL at 20 °C (lit.)
-
闪点:65-66°C/5mm
-
稳定性/保质期:
在常温常压下,该物质保持稳定。
计算性质
-
辛醇/水分配系数(LogP):5.24
-
重原子数:11
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:25.3
-
氢给体数:0
-
氢受体数:1
安全信息
-
TSCA:No
-
安全说明:S23,S24/25
-
危险类别码:R36/37/38
-
WGK Germany:3
-
储存条件:常温、避光、通风干燥处,密封保存。
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— bis(trimethylsilylmethyl)sulfoxide 108579-47-1 C8H22OSSi2 222.499
反应信息
-
作为反应物:描述:参考文献:名称:通过硫代羰基叶立德的[3+2]-环加成合成多官能化分子摘要:在这里,我们对硫代羰基叶立德与各种烯烃和炔烃的[3+2]-环加成进行了全面的研究。所获得的二氢和四氢噻吩产品作为用途极其广泛的中间体,提供了获得噻吩、二烯、树枝烯和八元碳中心的途径。高压条件的使用使得热不稳定、空间阻碍或中等反应性的底物能够在温和条件下进行环加成,从而将产率提高高达 58%。此外,我们还通过药物 NGB 4420 和替尼拉平的正式合成展示了其实用性。DOI:10.1021/jacs.9b07729
-
作为产物:参考文献:名称:通过硫代羰基叶立德的[3+2]-环加成合成多官能化分子摘要:在这里,我们对硫代羰基叶立德与各种烯烃和炔烃的[3+2]-环加成进行了全面的研究。所获得的二氢和四氢噻吩产品作为用途极其广泛的中间体,提供了获得噻吩、二烯、树枝烯和八元碳中心的途径。高压条件的使用使得热不稳定、空间阻碍或中等反应性的底物能够在温和条件下进行环加成,从而将产率提高高达 58%。此外,我们还通过药物 NGB 4420 和替尼拉平的正式合成展示了其实用性。DOI:10.1021/jacs.9b07729
文献信息
-
Electrooxidative B−H Functionalization of <i>nido</i> ‐Carboranes作者:Meng Chen、Deshi Zhao、Jingkai Xu、Chunxiao Li、Changsheng Lu、Hong YanDOI:10.1002/anie.202015299日期:2021.3.29of nido‐carboranes (7,8‐nido‐C2B9H12−) has been developed under electrochemical reaction conditions. In this reaction system, anodic oxidation serves as a green alternative for traditional chemical oxidants in the oxidation of nido‐carboranes. No transition‐metal catalyst is required and different heteroatoms bearing a lone pair are reactive in this transformation. Coupling nido‐carboranes with thioethers
-
Synthetic Applications of 3,4-Bis(trimethylsilyl)thiophene: Unsymmetrically 3,4-Disubstituted Thiophenes and 3,4-Didehydrothiophene<sup>,</sup>作者:Xin-Shan Ye、Henry N. C. WongDOI:10.1021/jo962191n日期:1997.4.1modification of 3,4-dibromothiophene; and (c) intermolecular thiazole-alkyne Diels-Alder reaction. 3,4-Bis(trimethylsilyl)thiophene (1a) can function as a versatile building block for the construction of unsymmetrically 3,4-disubstituted thiophenes utilizing its stepwise regiospecific mono-ipso-substitution followed by palladium-catalyzed cross-coupling reactions. In this manner, thiophenes 15, 16, 17a-j3,4-双(三甲基甲硅烷基)噻吩(1a)通过三种途径合成:(a)1,3-偶极环加成;(b)3,4-二溴噻吩的修饰;(c)分子间噻唑-炔Diels-Alder反应。3,4-双(三甲基甲硅烷基)噻吩(1a)可利用其逐步的区域特异性单-ipso取代,然后进行钯催化的交叉偶联反应,来构建不对称的3,4-二取代的噻吩。以这种方式,制备了噻吩15、16、17a-j,19a,b,20、22a-c,23a,b,24a-d,25a-c和27a-j。噻吩-3,4-二基二聚体28和噻吩-3,4-二基四聚体29也是通过钯催化的有机硼氧烷自偶联反应实现的。苯乙烯基噻吩31,通过经由环硼氧烷26c将C-Si键转化为C-Sn键而形成的化合物进行羰基化偶联和锂化,然后用亲电试剂淬灭,从而也提供不对称的3,4-二取代的噻吩33和36a-c。此外,3,4-双(三甲基甲硅烷基)噻吩(1a)可以用作生成高应变环状枯烯3,4-di
-
Rapid Assembly of Tetrasubstituted Furans via Pummerer-Type Rearrangement作者:Franz-Lucas Haut、Christoph Habiger、Lukas A. Wein、Klaus Wurst、Maren Podewitz、Thomas MagauerDOI:10.1021/jacs.0c12194日期:2021.1.20as exceptionally versatile intermediates and were shown to participate in a series of valuable postmodifications. The fate of the initial sulfonium intermediate was investigated by mechanistic experiments, and computational studies revealed the existence of an unprecedented Pummerer-type rearrangement. The potential for organic synthesis is highlighted by the total synthesis of bisabolene sesquiterpenoids
-
Evolution of a Polyene Cyclization Cascade for the Total Synthesis of (−)-Cyclosmenospongine作者:Klaus Speck、Thomas MagauerDOI:10.1002/chem.201605029日期:2017.1.23We report a full account on the development of a unique cationic polyene cyclization for the total synthesis of the tetracyclic meroterpenoid (−)‐cyclosmenospongine. A highly convergent three‐component coupling strategy enabled rapid access to individual cyclization precursors that were tested for their reactivity. The successful transformation generates three rings and sets four consecutive stereocenters我们报告了一个完整的发展为四环甲氧萜(-)-cyclosmenospongine的全合成独特的阳离子多烯环化的完整说明。高度收敛的三组分偶联策略使得能够快速访问经过测试其反应性的各个环化前体。成功的转换生成了三个环,并在一次操作中以高效的方式设置了四个连续的立体中心,从而仅给出了十萘烷的反式构架。此外,我们发现烯醇醚的几何形状以及C3和C8的相对构型对于多烯环化的成功至关重要。
-
Complexes of tin(IV) halides and organotin(IV) halides with organic sulphides and selenides: the low temperature cessation of sulphur and selenium inversion and its structural consequences作者:Edward W. Abel、Suresh K. Bhargava、Keith G. Orrell、Vladimir SikDOI:10.1016/s0020-1693(00)90454-7日期:1981.1characteristisation of the 1:2 complexes [SnX 4 (R 2 E) 2 ] (R = C 6 H 5 CH 2 , Me 3 SiCH 2 ; E = S, Se; X = Cl, Br) and the 1:1 complexes [SnCl 4 (bmt)] (bmt = 3,4-bis(methylthio)-toluene and [SnCl 3 (C 6 H 5 )(dth)] (dth = 2,5-dithiahexane). Variable temperature 1 H NMR studies have confirmed the previously observed cis/trans isomerism about tin in the [SnX 4 L 2 ] complexes, and we have additionally
表征谱图
-
氢谱1HNMR
-
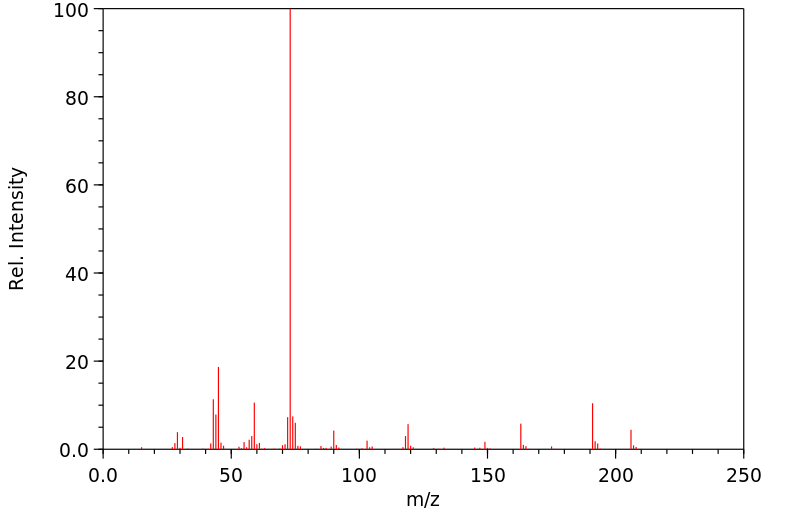
质谱MS
-
碳谱13CNMR
-
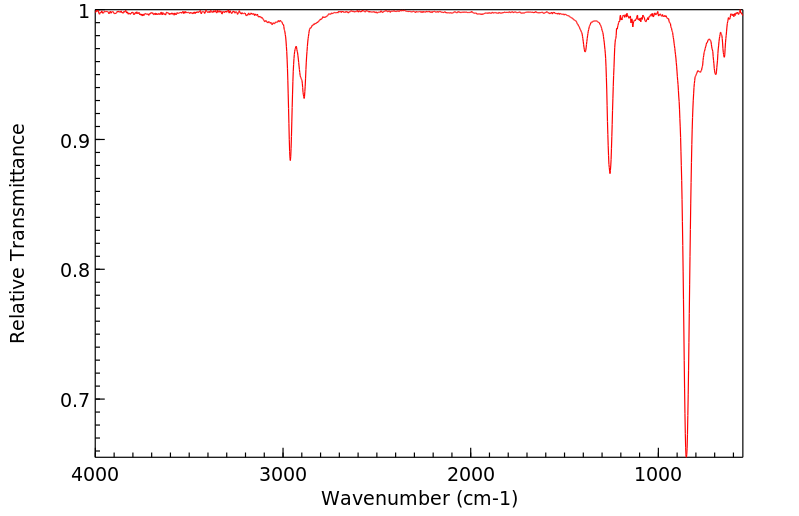
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄原酸环癸酯
高纯三甲基锑
顺式-二氯二(环丙胺)铂(II)
顺式-二氯二(乙二胺)氯化铑(1+)
顺式-二(环己基丁氨合)二氯铂(II)
顺式-二(异丙基氨合)二氯铂(II)
顺式-(2-氨基甲基-1-环戊基氨合)二氯铂(II)
顺二氯二羰基铂(II)
顺-二氯双(乙二胺)氯化铱
雷(酸)汞[含水或水加乙醇≥20]
间碳硼烷-9-硫醇
镍,加合(7:2)钪
镉二(二戊基二硫代氨基甲酸盐)
镁,溴-6-庚烯基-
manganese carbide
butyl manganese bromide
锡烷,氯二环己基-
锡四丁醇
锑,(1:1)混合物和钪
锌叔-丁氧化物
锌,溴-1-丙烯基-,(E)-
锇,加合(2:1)钪
锆酸四丁酯
锂丁酯
锂4-异丙氧基-2-甲基-丁烷-2-醇
锂1-丁醇
锂(三氟甲基)乙炔化物
锂(3-氨基丙基)酰胺
铼五羰基碘化物
铼五羰基
银(I)2-羟基乙烷-1-硫醇盐
铯三氯三羰基锇
铬三乙二胺
铬,五羰基(环己胺)-,(OC-6-22)-
铬,二(乙酰腈)二氯-
铝,加合(3:1)钪
铜-乙二胺络合物
铜(II)乙二胺
铜(I)乙炔化物
铍,环戊-1,3-二烯,溴化
铊N,N-二正丁胺
铊,甲氧基二甲基-
铂(2+)二氯化3-甲基丁烷-1,2-二胺(1:1)
铁(3+)三(1-丁醇)
铁(2+)1,1'-(硫烷二基二-1,1-乙二基)二-2,4-环戊二烯化
铀,三甲基-
钾,[三(三甲基甲硅烷基)甲基]-
钴四异硫氰酸酯
钴,乙烷-1,2-二胺
钠辛基二硫代氨基甲酸酯