吡咯烷二硫代氨基甲酸烯丙酯 | 701-13-3
中文名称
吡咯烷二硫代氨基甲酸烯丙酯
中文别名
1-吡咯烷二硫代羧酸烯丙酯
英文名称
Allyl 1-pyrrolidinecarbodithioate
英文别名
prop-2-enyl pyrrolidine-1-carbodithioate
CAS
701-13-3
化学式
C8H13NS2
mdl
MFCD00059099
分子量
187.33
InChiKey
GDQCWIUHBJJDNL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:255.1±33.0 °C(Predicted)
-
密度:1.14
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:11
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.625
-
拓扑面积:60.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2933990090
反应信息
-
作为反应物:描述:参考文献:名称:(E)-1,3-二烯和(E,E)-1,3,5-三烯的高度立体和区域选择性合成摘要:(E)-1-,3-dodecadien-1-ylacetate(红铃虫蛾的昆虫性信息素)和(E,E)-1,3.5-十一碳三烯(双翅目和Galbanum的成分)以高度立体合成烯丙基和戊二烯基二硫代氨基甲酸酯的-和区域选择性方式。DOI:10.1016/s0040-4039(00)87972-1
-
作为产物:描述:sodium pyrrolidine-N-carbodithioate 以78%的产率得到参考文献:名称:FAID-ALLAH, HASSAN M., REV. ROUM. CHIM., 33,(1988) N, C. 815-822摘要:DOI:
文献信息
-
An efficient synthesis of dithiocarbamates under ultrasound irradiation in water作者:Najmadin Azizi、Elahm Gholibeglo、Sanaz Dehghan NayeriDOI:10.1007/s00706-011-0687-z日期:2012.8economic, and green method for the synthesis of dithiocarbamate derivatives in water. The one-pot, three-component condensation of primary and secondary amines with carbon disulfide and unsaturated carbonyl compounds or alkyl halides under ultrasonic irradiation was developed as a green and fast protocol for the rapid high-yielding preparation of dithiocarbamates in water. Graphical abstract
-
Straightforward and Highly Efficient Catalyst-Free One-Pot Synthesis of Dithiocarbamates under Solvent-Free Conditions作者:Najmedin Azizi、Fezzeh Aryanasab、Mohammad R. SaidiDOI:10.1021/ol0620141日期:2006.11.9[Structure: see text] A highly efficient and simple synthesis of dithiocarbamates is possible based on the one-pot reaction of amines, CS2, and alkyl halides without using a catalyst under solvent-free conditions. The mild reaction conditions, high yields, and broad scope of the reaction illustrate the good synthetic utility of this method. The reaction is a highly atom-economic process for production
-
Allyl dithiocarbamate as a new β-acyl vinyl anion equivalent作者:Toshio HayashiDOI:10.1016/s0040-4039(00)97568-3日期:1990.1The title compound can function as synthetic equivalents of β-formyl vinyl and β-halomethyl vinyl anions, as shown by the conversion of aldehydes to 4-hydroxy-2(E)-enals and 4-hydroxy-1-iodo-2(E)-alkenes.
-
A NEW, GENERAL, AND STEREOSELECTIVE SYNTHESIS OF LONG CHAIN TETRAENOIC ACIDS EXAMPLIFIED BY β-PARINARIC ACID作者:Toshio Hayashi、Takeshi OishiDOI:10.1246/cl.1985.413日期:1985.3.5All trans-9,11,13,15-octadecatetraenoic acid was first synthesized in a stereoselective manner, using pentadienyl and allyl dithiocarbamates as the starting materials. This synthesis confirmed that β-parinaric acid has all trans configurations about the four double bonds.
-
Regiospecific iodocyclization of S-allyl dithiocarbamates: synthesis of 2-imino-1,3-dithiolane and 2-iminium-1,3-dithiolane derivatives作者:Azim Ziyaei Halimehjani、Hajar Maleki、Mohammad R. SaidiDOI:10.1016/j.tetlet.2009.03.127日期:2009.64-Alkyl-2-imino-1,3-dithiolanes and 4-alkyl-2-iminium-1,3-dithiolanes were prepared in excellent yields with complete regiospecificity under mild conditions by the iodocyclization of S-allyl dithiocarbamates. Dehydrohalogentaion of the 4-alkyl-2-imino-1,3-dithiolanes gave 4-alkylidene-2-imino-1,3-dithiolanes in excellent yields. (C) 2009 Elsevier Ltd. All rights reserved.
表征谱图
-
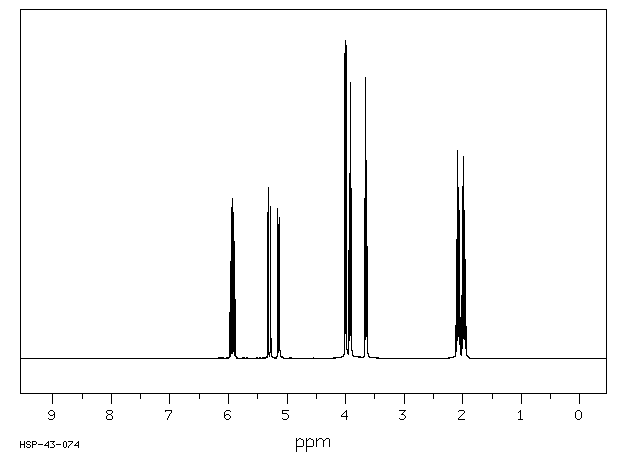
氢谱1HNMR
-
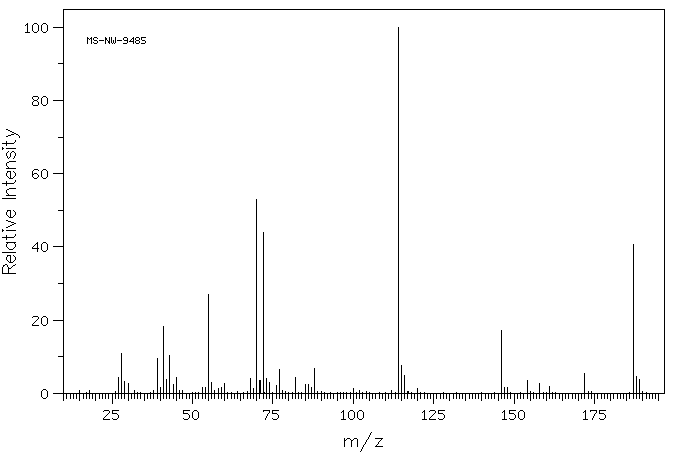
质谱MS
-
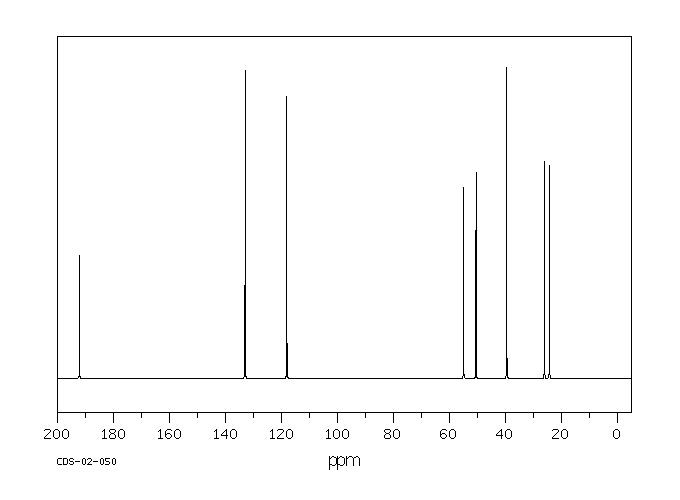
碳谱13CNMR
-
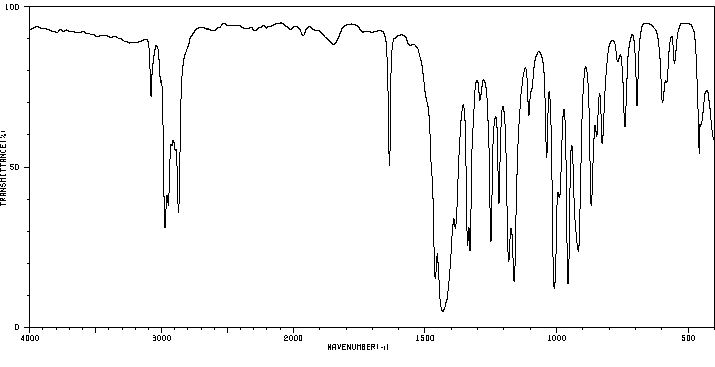
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5R,Z)-3-(羟基((1R,2S,6S,8aS)-1,3,6-三甲基-2-((E)-prop-1-en-1-yl)-1,2,4a,5,6,7,8,8a-八氢萘-1-基)亚甲基)-5-(羟甲基)-1-甲基吡咯烷-2,4-二酮
(2R,2''R)-(-)-2,2''-联吡咯烷
麦角甾-7,22-二烯-3-基亚油酸酯
马来酰亚胺霉素
马来酰亚胺基酰肼盐酸盐
马来酰亚胺基甲基-3-马来酰亚胺基丙酸酯
马来酰亚胺丙酰基-dPEG4-NHS
马来酰亚胺-酰胺-PEG6-琥珀酰亚胺酯
马来酰亚胺-酰胺-PEG6-丙酸
马来酰亚胺-酰胺-PEG24-丙酸
马来酰亚胺-酰胺-PEG12-丙酸
马来酰亚胺-四聚乙二醇-羧酸
马来酰亚胺-四聚乙二醇-丙酸叔丁酯
马来酰亚胺-四聚乙二醇-丙烯酸琥珀酰亚胺酯
马来酰亚胺-六聚乙二醇-羧酸
马来酰亚胺-六聚乙二醇-丙酸叔丁酯
马来酰亚胺-八聚乙二醇-丙酸叔丁酯
马来酰亚胺-二聚乙二醇-丙酸叔丁酯
马来酰亚胺-三(乙烯乙二醇)-丙酸
马来酰亚胺-一聚乙二醇-羧酸
马来酰亚胺-一聚乙二醇-丙烯酸琥珀酰亚胺酯
马来酰亚胺-PEG3-羟基
马来酰亚胺-PEG2-胺三氟醋酸盐
马来酰亚胺-PEG2-琥珀酰亚胺酯
马来酰亚胺
频哪醇硼酸酯
顺式草酸双(-3,8-二氮杂双环[4.2.0]辛烷-8-羧酸叔丁酯)
顺式4-甲基吡咯烷酮-3-醇盐酸盐
顺式4-氟吡咯烷酮-3-醇盐酸盐
顺式3,4-二羟基吡咯烷盐酸盐
顺式3,4-二氨基吡咯烷-1-羧酸叔丁酯
顺式-二甲基 1-苄基吡咯烷-3,4-二羧酸
顺式-N-[2-(2,6-二甲基-1-哌啶基)乙基]-2-氧代-4-苯基-1-吡咯烷乙酰胺
顺式-N-Boc-吡咯烷-3,4-二羧酸
顺式-5-苄基-2-叔丁氧羰基六氢吡咯并[3,4-c]吡咯
顺式-5-甲基-1H-六氢吡咯并[3,4-b]吡咯二盐酸盐
顺式-5-氧代六氢环戊二烯并[c]吡咯-2(1H)-羧酸叔丁酯
顺式-5-乙氧羰基-1H-六氢吡咯并[3,4-B]吡咯盐酸盐
顺式-5-(碘甲基)-4-苯基-2-吡咯烷酮
顺式-5-(碘甲基)-4-甲基-2-吡咯烷酮
顺式-4-氧代-六氢-吡咯并[3,4-C]吡咯-2-甲酸叔丁酯
顺式-3-氟-4-羟基吡咯烷-1-羧酸叔丁酯
顺式-3-氟-4-甲基吡咯烷盐酸盐
顺式-2-甲基六氢吡咯并[3,4-c]吡咯
顺式-2,5-二甲基吡咯烷
顺式-1-苄基-3,4-吡咯烷二甲酸二乙酯
顺式-1-甲基六氢吡咯并[3,4-b]吡咯
顺式-(9CI)-3,4-二乙烯-1-(三氟乙酰基)-吡咯烷
顺-八氢环戊[c]吡咯-5-酮盐酸盐
非星匹宁