四甲基萘-1,2,3,4-四羧酸酯 | 36063-07-7
中文名称
四甲基萘-1,2,3,4-四羧酸酯
中文别名
——
英文名称
tetramethyl naphthalene-1,2,3,4-tetracarboxylate
英文别名
1,2,3,4-Tetramethoxycarbonyl-naphthalin;Tetramethyl 1,2,3,4-naphthalenetetracarboxylate
CAS
36063-07-7
化学式
C18H16O8
mdl
——
分子量
360.32
InChiKey
WJUUSKXVCZGLRD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:147 °C
-
沸点:428.2±40.0 °C(Predicted)
-
密度:1.309±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:26
-
可旋转键数:8
-
环数:2.0
-
sp3杂化的碳原子比例:0.22
-
拓扑面积:105
-
氢给体数:0
-
氢受体数:8
SDS
反应信息
-
作为反应物:描述:四甲基萘-1,2,3,4-四羧酸酯 在 sodium hydroxide 作用下, 以 四氢呋喃 、 水 为溶剂, 生成 萘-四羧酸 、 1,3-Dioxobenzo[e][2]benzofuran-4,5-dicarboxylic acid参考文献:名称:1,2,3,4-萘和蒽二酰亚胺的合成与表征摘要:抽象的 我们报告了由环状酰亚胺封端的萘和蒽支架的合成和表征。通过 X 射线晶体学测定的N-苯基衍生物的固态结构揭示了基于核中芳环数量的堆积偏好的变化。标题化合物的光学和电子性质与之前描述的其他异构体相比毫不逊色,并扩展了有机材料化学家可用的缺电子芳香族化合物的工具箱。 Beilstein J. Org. Chem. 2024, 20, 1767–1772. doi:10.3762/bjoc.20.155DOI:10.3762/bjoc.20.155
-
作为产物:参考文献:名称:Coupling of Internal Alkynes in TpMe2Ir Derivatives: Selective Oxidation of a Noncoordinated Double Bond of the Resulting Iridacycloheptatrienes摘要:The reaction of different Tp(Me2)Ir derivatives and dimethylacetylene dicarboxylate (DMAD) allows the preparation of three different metallacycloheptatriene complexes and an unusual allyl-terminated metallacycle. The C atoms of distant C=C bonds in the metallacycles, including aromatic ones, can be converted selectively to the corresponding keto functionality under mild conditions.DOI:10.1021/ja0290375
文献信息
-
O-Dihaloarenes as aryne precursors for nickel-catalyzed [2 + 2 + 2] cycloaddition with alkynes and nitriles作者:Jen-Chieh Hsieh、Chien-Hong ChengDOI:10.1039/b801870g日期:——o-Dihaloarenes acting as aryne precursors react with acetylenes and nitriles catalyzed by the NiBr(2)(dppe)/dppe/Zn system to give substituted naphthalene, phenanthridine or triphenylene derivatives depending on the reaction conditions in moderate to excellent yields with good tolerance of functional groups.
-
Reaction cascades initiated by nucleophilic attack of heteropentalene mesomeric betaine and nitrogen-rich mesoionic tetrazolium-5-amides on electron-deficient unsaturated compounds. Synthesis of novel heterocyclic systemsElectronic supplementary information (ESI) available: synthesis of 4b; reactions of 11 and 17 with electron-deficient unsaturated compounds; alternative synthesis of 19 from 20. See http://www.rsc.org/suppdata/ob/b2/b211000h/作者:Shuki Araki、Masashi Kuzuya、Kenji Hamada、Masatoshi Nogura、Nayumi OhataDOI:10.1039/b211000h日期:2003.3.13The reactions of heteropentalene mesomeric betaine 1 and nitrogen-rich mesoionic tetrazolium-5-amides 4, 11 and 16-18 with electron-deficient unsaturated compounds have been studied. Novel heterocyclic systems, tetrazolo[4,5-a][1,7]benzodiazonine inner salt 2 and 3-oxo-3,7-dihydro-2H-pyrazolo[3,4-b]pyridine 5, have been synthesized by the reactions of dimethyl acetylenedicarboxylate with 1 and tetrazolium-5-anilide
-
PALLADIUM-PROMOTED AROMATIC ANNELATION WITH ACETYLENE DICARBOXYLATES作者:Tsutomu Sakakibara、Yasuo Tanaka、Shin-ichi YamasakiDOI:10.1246/cl.1986.797日期:1986.5.5Benzene rings of N-alkyltoluidine and benzene (or iodobenzene) in acetic acid were annelated with acetylenedicarboxylates by palladium(II) acetate to give N-alkylindole-2,3-dicarboxylate and naphthalene-1,2,3,4-tetracarboxylate. The formation of the products are explained in terms of toluidine radical cation intermediates.
-
Selective Palladium-Catalyzed Cocyclotrimerization of Arynes with Dimethyl Acetylenedicarboxylate: A Versatile Method for the Synthesis of Polycyclic Aromatic Hydrocarbons作者:Diego Peña、Dolores Pérez、Enrique Guitián、Luis CastedoDOI:10.1021/jo000535a日期:2000.10.1derivatives 4,5-difluorobenzyne (1b) and 3-methoxybenzyne (2) undergo chemoselective palladium-catalyzed [2 + 2 + 2]-cocyclotrimerization with dimethyl acetylenedicarboxylate (DMAD) to afford the corresponding phenanthrenes and/or naphthalenes. The major products are phenanthrenes if Pd(PPh(3))(4) is used as the catalyst, naphthalenes if Pd(2)(dba)(3) is used. When the method is applied to polycyclic arynes
-
Synthesis of Tetrasubstituted Naphthalenes by Palladium-Catalyzed Reaction of Aryl Iodides with Internal Alkynes作者:Satoshi Kawasaki、Tetsuya Satoh、Masahiro Miura、Masakatsu NomuraDOI:10.1021/jo0346656日期:2003.8.1acetylenedicarboxylate esters and diphenylacetylene efficiently proceeds in the presence of a palladium catalyst with use of silver carbonate as base to produce the corresponding tetrasubstituted naphthalenes.
表征谱图
-
氢谱1HNMR
-
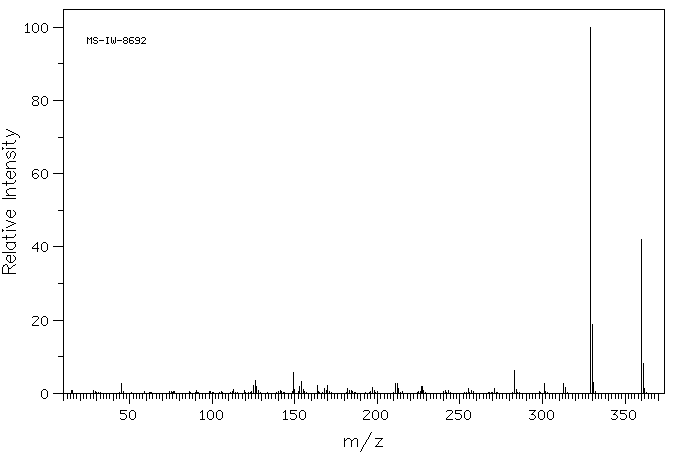
质谱MS
-
碳谱13CNMR
-
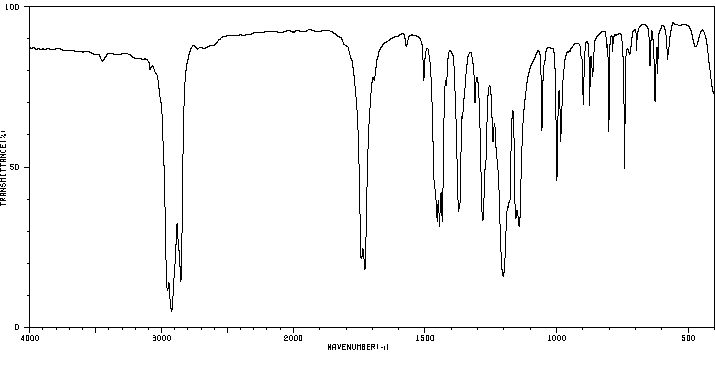
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮