(二甲氨基)乙腈 | 926-64-7
中文名称
(二甲氨基)乙腈
中文别名
N,N-二甲基氨基乙腈;2-(二甲胺基)乙腈;N,N-二甲胺基乙氰;N,N-二甲氨基乙腈;N,N-甲基氨基乙腈;N,N-二甲基氨基乙腈;2-(二甲氨基)乙腈
英文名称
N,N-Dimethylamino acetonitrile
英文别名
(dimethylamino)acetonitrile;2-(dimethylamino)acetonitrile;Dimethylaminoacetonitrile
CAS
926-64-7
化学式
C4H8N2
mdl
MFCD00001890
分子量
84.1209
InChiKey
PLXBWEPPAAQASG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
常温常压下稳定,避免与强氧化剂和酸接触。
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:27
-
氢给体数:0
-
氢受体数:2
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2-(dimethylamino)malononitrile 19555-13-6 C5H7N3 109.131 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 二甲基十二/十四烷基叔胺 N,N-dimethyl-ethanamine 598-56-1 C4H11N 73.138
反应信息
-
作为反应物:描述:参考文献:名称:DE694808摘要:公开号:
-
作为产物:参考文献:名称:The evidence for generation of dimethylaminocyanocarbene in the thermolysis of dimethylaminomalononitrile. The dimethylamino(dicyano- and cyano)methyl radicals, carbon analogs of the nitroxides摘要:DOI:10.1021/ja00471a045
-
作为试剂:描述:参考文献:名称:Larkin, Finbar; O'Ferrall, Rory A.More, Australian Journal of Chemistry, 1983, vol. 36, # 9, p. 1831 - 1842摘要:DOI:
文献信息
-
Die präparative Chemie der<i>O</i>- und<i>N</i>-funktionellen Orthokohlensäure-Derivate作者:W. KANTLEHNER、B. FUNKE、E. HAUG、P. SPEH、L. KIENITZ、T. MAIERDOI:10.1055/s-1977-24283日期:——The methods for the preparation of O- and N-functional orthocarbonic acid derivatives are reviewed, and a survey of the reactions of these compounds with electrophilic and nucleophilic reagents is given. 1. Synthesis of Orthocarbonic Acid Esters 1.1 Reaction of Substituted Trichloromethanes with Alcohols, Alkoxides, Phenols, or Phenoxides 1.2. Reaction of Substituted Dichloromethanes with Alcohols or Phenols 1.3. Reaction of Trialkoxycarbenium Salts with Alkoxides 1.4. Reaction of Cyanic Acid Esters with Alcohols 1.5. Thermolysis of Cyclic Carbonic Acid Derivatives 1.6. Reaction of Carbon Disulfide with Thallium(I) Alkoxides 1.7. Reaction of Carbon Disulfide with Organotin Compounds 1.8. Reaction of Zinc Xanthates with Alcohols 1.9. Transesterification of Orthocarbonic Acid Esters 2. Synthesis of Orthocarbamic Acid Esters 2.1. Reaction of Trihetero-substituted Carbenium Ions with Alkoxides 2.2. Alcoholysis of Tetramethylurea Diethyl Acetal 2.3. Transesterification of Orthocarbamic Acid Esters 2.4. Spirocyclic Orthocarbamic Acid Esters from Organotin Compounds 3. Synthesis of Bis[dialkylamino]-dialkoxymethanes (Tetraalkylurea Dialkyl Acetals) 3.1. Reaction of Bis[dialkylamino]-ethoxycarbenium Tetrafluoroborates with Alkoxides 3.2. Reaction of Tetraalkylurea Dichlorides (Chloroformamidinium Chlorides) with Alcohols or Alkoxides 3.3. Reaction of 2,2-Dichloro-1,3-benzodioxole with Amines 3.4. Spirocyclic Urea Acetals from Organotin Compounds 4. Synthesis of Alkoxytris[dialkylamino]methanes 5. Synthesis of Tetrakis[dialkylamino]methanes 6. Reactions of O- and N-Functional Orthocarbonic Acid Derivatives 6.1. Reactions with Electrophilic Agents 6.2. Reactions with Nucleophilic Agents 6.3. Miscellaneous Reactions of Orthocarbonic Acid Derivatives综述了O-和N-功能性原碳酸衍生物的制备方法,并介绍了这些化合物与亲电试剂和亲核试剂的反应。 1. 原碳酸酯的合成 1.1. 取代三氯甲烷与醇、醇盐、酚或酚盐的反应 1.2. 取代二氯甲烷与醇或酚的反应 1.3. 三烷氧基碳鎓盐与醇盐的反应 1.4. 氰酸酯与醇的反应 1.5. 环状碳酸衍生物的热解 1.6. 二硫化碳与铊(I)烷氧基化合物的反应 1.7. 二硫化碳与有机锡化合物的反应 1.8. 锌黄原酸盐与醇的反应 1.9. 原碳酸酯的酯交换反应 2. 原氨基甲酸酯的合成 2.1. 三杂取代碳鎓离子与醇盐的反应 2.2. 四甲基脲二乙缩醛的醇解 2.3. 原氨基甲酸酯的酯交换反应 2.4. 有机锡化合物制备的螺环原氨基甲酸酯 3. 双[二烷基氨基]-二烷氧基甲烷(四烷基脲二烷基缩醛)的合成 3.1. 双[二烷基氨基]-乙氧基碳鎓四氟硼酸盐与醇盐的反应 3.2. 四烷基脲二氯化物(氯仿酰亚胺氯化物)与醇或醇盐的反应 3.3. 2,2-二氯-1,3-苯并二氧杂环戊烯与胺的反应 3.4. 有机锡化合物制备的螺环脲缩醛 4. 烷氧基三[二烷基氨基]甲烷的合成 5. 四[二烷基氨基]甲烷的合成 6. O-和N-功能性原碳酸衍生物的反应 6.1. 与亲电试剂的反应 6.2. 与亲核试剂的反应 6.3. 原碳酸衍生物的其他反应
-
Chlorine dioxide oxidation of amines: synthetic utility and a biomimetic synthesis of elaeocarpidine作者:Chien Kuang. Chen、Alfred G. Hortmann、Mohammad R. MarzabadiDOI:10.1021/ja00222a052日期:1988.7Oxydation par ClO 2 d'amino-3 propanols-1 et d'ethanolamines et cyanation oxydante d'amines tertiaires, la fonction amine pouvant etre un heterocycle azote氧化 par ClO 2 d'amino-3 propanols-1 et d'ethanolamines et cyanation oxydante d'amines tertiaires, la fonction amine pouvant etre unheterocycle azote
-
Anthelmintic 1-(substituted phenyl)-3-alkanimidoyl ureas申请人:Sterling Drug Inc.公开号:US03984467A1公开(公告)日:1976-10-05The compounds of this invention are novel imidoylureas having anthelmintic activity and imidoylthioureas having antifertility activity. They are prepared by the reaction of appropriate amidines with appropriate isocyanates or isothiocyanates.
-
Novel O-acylated amidoximes and substituted 1,2,4-oxadiazoles synthesised from (+)-ketopinic acid possessing potent virus-inhibiting activity against phylogenetically distinct influenza A viruses作者:Vladimir V. Chernyshov、Olga I. Yarovaya、Iana L. Esaulkova、Ekaterina Sinegubova、Sophia S. Borisevich、Irina I. Popadyuk、Vladimir V. Zarubaev、Nariman F. SalakhutdinovDOI:10.1016/j.bmcl.2021.128465日期:2022.1and for antiviral activity against influenza viruses of H1N1 and H7N9 subtypes. The synthesised compounds exhibited high virus-inhibiting activity against the H1N1 influenza virus. Some synthesised compounds were also active against the influenza virus of a different antigenic subtype: H7N9. The mechanism of the virus-inhibiting activity of these compounds is based on their interference with the fusion本文介绍了在杂环的第 5 位含有双环取代基的新型取代 1,2,4-恶二唑和 O-酰化偕胺肟作为其合成前体的合成和抗病毒活性评估。从(+)-樟脑衍生物(+)-酮辛酸中获得了新的化合物。化学文库进行了体外测试对 MDCK 细胞系的细胞毒性和对 H1N1 和 H7N9 亚型流感病毒的抗病毒活性。合成的化合物对 H1N1 流感病毒表现出高病毒抑制活性。一些合成的化合物对不同抗原亚型的流感病毒也有活性:H7N9。这些化合物的病毒抑制活性机制是基于它们干扰病毒血凝素(HA)的融合活性。已证明对 HA 的受体结合活性没有干扰。根据分子对接结果,O-酰化偕胺肟和1,2,4-恶二唑的选择性抗病毒活性与其结构特征有关。O-酰化偕胺肟可能与位于融合肽位点的结合位点更互补,并且 1,2, 4-恶二唑与位于蛋白水解位点的位点更互补。不同类型 HA 结合位点的氨基酸残基的显着差异使我们能够解释所研究化合物的选择性抗病毒活性。
-
Decarbonylative Synthesis of Aryl Nitriles from Aromatic Esters and Organocyanides by a Nickel Catalyst作者:Junichiro Yamaguchi、Keiichiro Iizumi、Miki B. Kurosawa、Ryota Isshiki、Kei MutoDOI:10.1055/s-0040-1705943日期:2021.9A decarbonylative cyanation of aromatic esters with aminoacetonitriles in the presence of a nickel catalyst was developed. The key to this reaction was the use of a thiophene-based diphosphine ligand, dcypt, permitting the synthesis of aryl nitrile without the generation of stoichiometric metal- or halogen-containing chemical wastes. A wide range of aromatic esters, including hetarenes and pharmaceutical
表征谱图
-
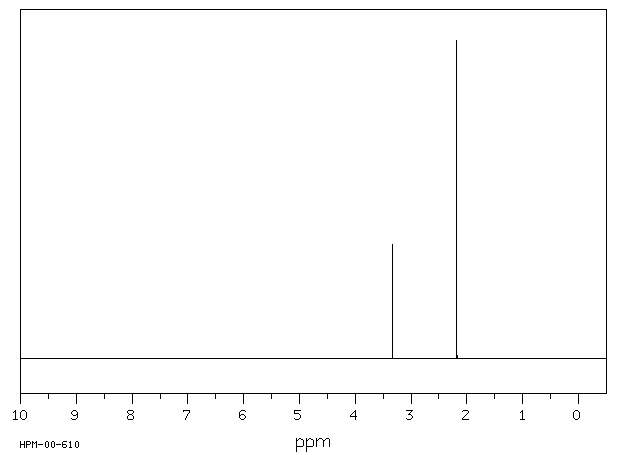
氢谱1HNMR
-
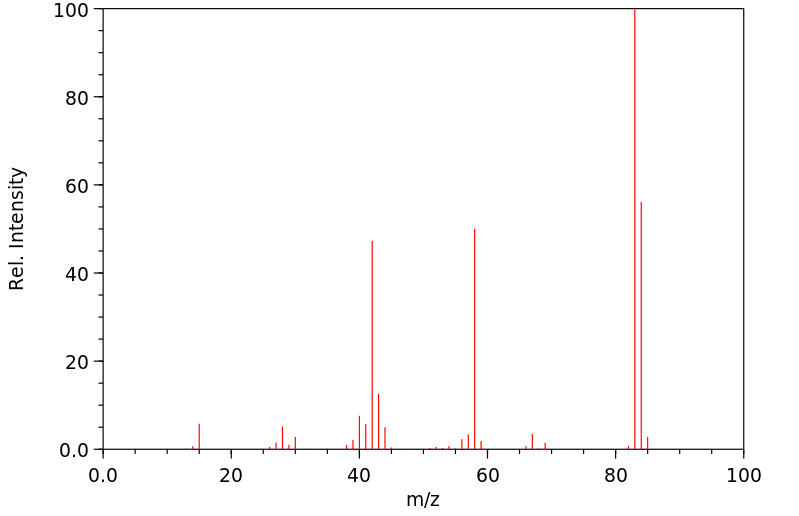
质谱MS
-
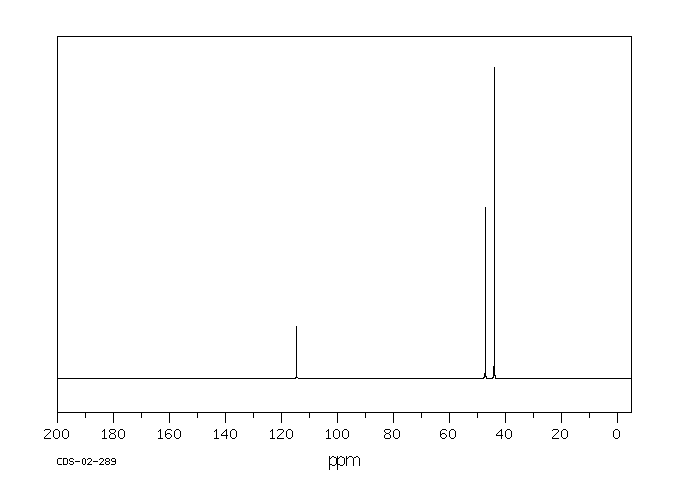
碳谱13CNMR
-
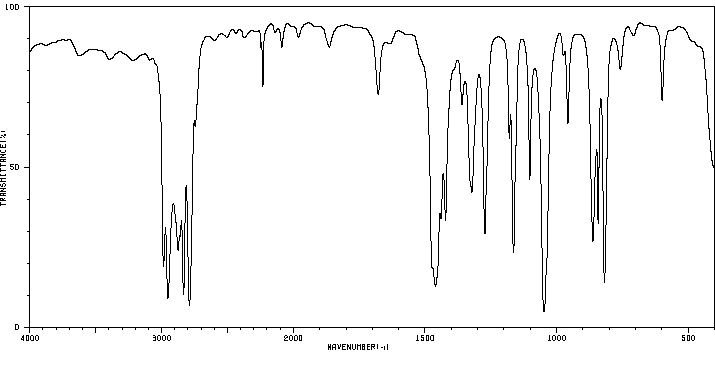
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷