1-(4-氯苯硫基)-2-丙醇 | 13663-04-2
中文名称
1-(4-氯苯硫基)-2-丙醇
中文别名
——
英文名称
(RS)-1-(p-chlorophenyl)thiopropan-2-ol
英文别名
1-((4-chlorophenyl)thio)propan-2-ol;1-(4'-chlorophenylthio)propan-2-ol;1-p-Chlorphenylthio-2-propanol;1-(p-Chlorophenylthio)-2-propanol;1-(4-chlorophenyl)sulfanylpropan-2-ol
CAS
13663-04-2
化学式
C9H11ClOS
mdl
——
分子量
202.705
InChiKey
CHHYHUZLMAKWAS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:45.5
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2930909090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (4-氯苯基硫)丙-2-酮 (p-chlorophenylthio)acetone 25784-83-2 C9H9ClOS 200.689
反应信息
-
作为反应物:描述:1-(4-氯苯硫基)-2-丙醇 、 (R)-methoxytrifluoromethylphenylacetyl chloride 在 吡啶 、 4-二甲氨基吡啶 作用下, 以 二氯甲烷 为溶剂, 反应 16.0h, 生成参考文献:名称:Rhizopus arrhizus-mediated asymmetric reduction of arylalkanones: unusual anti-Prelong products with benzyl alkyl ketones摘要:Rhizopus arrhizus-mediated microbial reduction of various aryl alkyl ketones afforded chiral carbinols in good yields and high enantiomeric purity. The most striking feature was the formation of the anti-Prelog (R)-alcohols with the benzyl alkyl ketones, while the other ketones ArXCOR (X = (CH2)(n), n = 0 or 2, OCH2 or SCH2 and R = Me/Et/n-Bu) furnished (S)-alcohols. (C) 2011 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetasy.2011.08.015
-
作为产物:描述:(4-氯苯基硫)丙-2-酮 在 甲醇 、 sodium tetrahydroborate 作用下, 反应 0.5h, 以95%的产率得到1-(4-氯苯硫基)-2-丙醇参考文献:名称:用于通过酮还原酶在 C-O 或 C-S 键处具有立体中心的 β-羟基硫化物的对映选择性合成的化学酶级联反应摘要:鉴定了四种酮还原酶 (KRED) 用于 β-羟基硫化物的对映选择性合成。KRED311 和 KRED349 通过苯硫酚/硫醇和 α-卤代酮/醇的化学酶级联催化合成在 C-O 键处具有立体中心且绝对构型相反的 β-羟基硫化物。KRED253 和 KRED384 通过外消旋 α-硫代醛的动态动力学拆分 (DKR) 催化合成在 C-S 键处具有立体中心并具有相反对映选择性的 β-羟基硫化物。DOI:10.1002/anie.202202363
文献信息
-
Efficient Synthesis of <font>β</font>-Hydroxy Sulfides and <font>β</font>-Hydroxy Sulfoxides Catalyzed by Cu/MgO Under Solvent-Free Conditions作者:Biswanath Das、Penagaluri Balasubramanyam、Maddeboina Krishnaiah、Boyapati Veeranjaneyulu、Dega SudhakarDOI:10.1080/00397910903219500日期:2010.6.25Regio-, stereo-, and chemoselective ring opening of epoxides with thiols using Cu/MgO as a heterogeneous catalyst has efficiently been carried out to produce the corresponding β-hydroxy sulfides in excellent yields at room temperature under solvent-free conditions. The treatment of the epoxides with thiols and 50% aqueous H2O2 in the presence of the same catalyst at room temperature affords the β-hydroxy
-
Kinetic Resolution of β- and γ-Hydroxy Sulfides by Fungal Lipase from Humicola lanuginosa作者:Satwinder Singh、Subodh Kumar、Swapandeep Singh ChimniDOI:10.1080/10242430212883日期:2002.7Racemic beta- and gamma-hydroxy sulfides were resolved by Humicola lanuginosa lipase catalyzed transesterification using vinyl acetate both as acyl donor and solvent. The effect of substituents and spacer length on rate of reaction and enantioselectivity is observed.
-
Regioselective Reaction of Epoxides with Disulfides Using Zn/AlCl<sub>3</sub>System: A Simple Synthesis of β-Hydroxy Sulfides作者:B. Movassagh、S. Sobhani、F. Kheirdoush、Z. FadaeiDOI:10.1081/scc-120023422日期:2003.9Abstract Epoxides are regioselectively cleaved by zinc thiolate intermediate, generated from disulfides in the presence of Zn/AlCl3 system, to afford high yields of the corresponding β-hydroxy sulfides.
-
Cytotoxic compounds. Part XIII. Some 1-arylthiopropan-2-ols and 2-arylthiopropanols. Rearrangement of the primary methanesulphonates into the secondary isomers作者:M. S. Khan、L. N. OwenDOI:10.1039/j39710001448日期:——naphthyl compounds. Except for the 2,4-dinitrophenyl derivative (prepared in the normal way with methanesulphonyl chloride) the secondary methanesulphonates could only be obtained by the use of methanesulphonic anhydride under special conditions. Of the primary alcohols, the 2,4-dinitrophenyl compound gave the primary methanesulphonate but all the others gave mainly the secondary methanesulphonates.
-
Complex catalyst, process for producing the complex catalyst, and process for producing alchohol derivative with the complex catalyst申请人:——公开号:US20040077487A1公开(公告)日:2004-04-22There are provided (asymmetric) complex catalysts comprising metal complexes and Lewis acids as components, the metal complex being of formula (1): 1 wherein R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 and R 8 are the same or different and are independently hydrogen, halogen, alkyl or the like; one of R 9 and R 10 is hydrogen and the other is alkyl of 1 to 4 carbon atoms or the like; Q is a single bond or alkylene of 1 to 4 carbon atoms; M is a metal ion; and A is a balancing counter ion or ligand; processes for the production of these complex catalysts; processes for the production of (optically active) alcohol derivatives, characterized in that cyclic ether compounds are reacted with phenol derivatives in the presence of these complex catalysts; and further processes for producing (optically active) nitrogen-containing heterocyclic compounds by reacting these alcohol derivatives with halogenated nitrogen-containing heterocyclic compounds in the presence of a base.
表征谱图
-
氢谱1HNMR
-
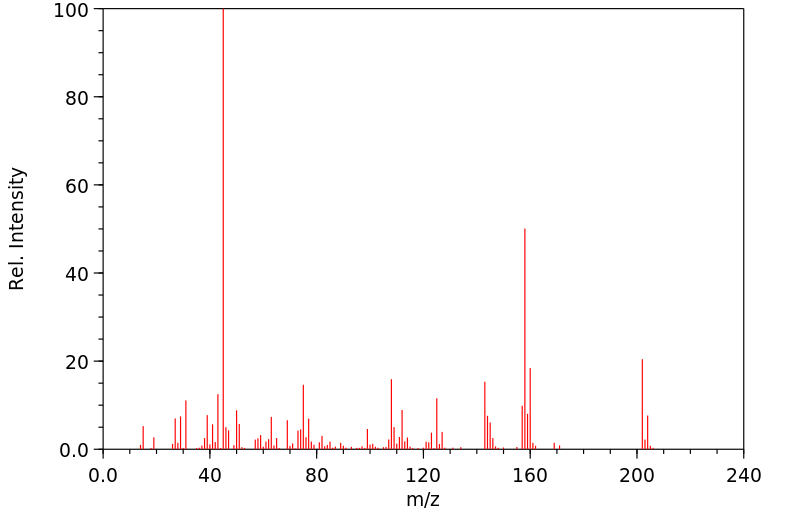
质谱MS
-
碳谱13CNMR
-
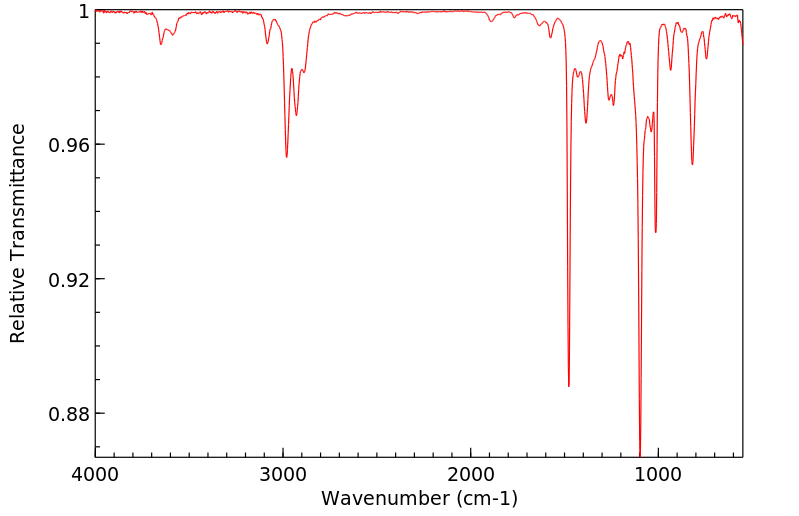
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯