1-二氮杂萘硫醇 | 16015-46-6
中文名称
1-二氮杂萘硫醇
中文别名
——
英文名称
1(2H)-phthalazinethione
英文别名
Phthalazine-1-thione;phthalazin-1-thione;2H-phthalazine-1-thione;2H-Phthalazin-1-thion;1(2H)-Phthalazinthion;Phthalazthion;2H-phthalazine-1-thione
CAS
16015-46-6
化学式
C8H6N2S
mdl
MFCD01918266
分子量
162.215
InChiKey
RGYHGSBVFWRLCJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:163-167°C
-
溶解度:溶于氯仿、二氯甲烷、DMSO、甲醇
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:56.5
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2933990090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,2-Dihydro-2-methyl-1-thio-phthalazin 20988-87-8 C9H8N2S 176.242
反应信息
-
作为反应物:描述:1-二氮杂萘硫醇 在 sodium hydroxide 、 乙醇 、 三乙胺 、 2,3-二氯-5,6-二氰基-1,4-苯醌 作用下, 以 1,4-二氧六环 、 乙醚 、 xylene 为溶剂, 反应 39.0h, 生成 3-phenylpyrrolo<2,1-a>phthalizine-1-carboxylic acid参考文献:名称:Annulation to the phthalazine ring system utilizing mesoionic ring systems摘要:DOI:10.1021/jo00210a022
-
作为产物:描述:参考文献:名称:Fujii; Sato, Tanabe seiyaku kenkyu nenpo = Annual report of Gohei Tanabe Co., 1956, vol. 1, # 1, p. 1,2摘要:DOI:
文献信息
-
[EN] HETEROCYCLIC COMPOUNDS THAT BLOCK THE EFFECTS OF ADVANCED GLYCATION END PRODUCTS (AGE)<br/>[FR] COMPOSES HETEROCYCLIQUES BLOQUANT LES EFFETS DES PRODUITS TERMINAUX AVANCES DE GLYCATION申请人:REDDYS LAB LTD DR公开号:WO2005040163A1公开(公告)日:2005-05-06The present invention relates to novel compounds of Formula (Ia) or (Ib), their pharmaceutically acceptable salts and pharmaceutically acceptable compositions containing them. Formula (Ia), or Formula (Ib) in the above formulae L represents Formula (I), Formula (II) or -(CH2)1- Q represents Formula (III), or Formula (IV). The present invention also relates to a process for the preparation of the above said novel compounds, their pharmaceutically acceptable salts and pharmaceutical compositions containing them. The present invention also relates to methods and compositions comprising compounds that treat pathophysiological conditions arising from inflammatory responses. In particular, the present invention is directed to compounds that inhibit or block glycated protein produced induction of the signaling-associated inflammatory responses in tissues, including endothelial cells. The present invention relates to compounds that inhibit smooth muscle proliferation. The present invention also relates to compounds that act as modulators of Perlecan activity and expression. In particular, the present invention is directed to compounds that inhibit smooth muscle cell proliferation by modulating heparan sulfate proteoglycans (HSPG) such as Perlecan. The present invention further relates to the use of compounds to treat vascular occlusive conditions characterized by smooth muscle proliferation such as stenosis, restenosis, neointimal hyperplasia, and atherosclerosis. The compounds of Formula (I) are also useful for the treatment and/or prevention of cancer. The types of cancers include melanoma, prostate, leukemia, lymphoma, non-small lung cancers, cancer of the central nervous system, breast, colon, ovarian or renal cancer.本发明涉及公式(Ia)或(Ib)的新化合物,它们的药学上可接受的盐以及含有它们的药学上可接受的组合物。在上述公式中,公式(Ia)或公式(Ib)中的L代表公式(I)、公式(II)或-(CH2)1-,Q代表公式(III)或公式(IV)。本发明还涉及制备上述新化合物、其药学上可接受的盐和含有它们的药物组合物的方法。本发明还涉及包括治疗由炎症反应引起的病理生理条件的化合物的方法和组合物。具体而言,本发明涉及抑制或阻断组织中糖化蛋白诱导的与信号相关的炎症反应的化合物。本发明涉及抑制平滑肌增殖的化合物。本发明还涉及作为Perlecan活性和表达调节剂的化合物。具体而言,本发明涉及通过调节肝素硫酸蛋白聚糖(HSPG)如Perlecan来抑制平滑肌细胞增殖的化合物。本发明进一步涉及利用化合物治疗由平滑肌增殖特征的血管闭塞性疾病,如狭窄、再狭窄、新内膜增生和动脉粥样硬化。公式(I)的化合物也可用于治疗和/或预防癌症。癌症类型包括黑色素瘤、前列腺癌、白血病、淋巴瘤、非小细胞肺癌、中枢神经系统癌症、乳腺癌、结肠癌、卵巢癌或肾癌。
-
Photoaddition of Olefins to Quinolin-, Isoquinolin-, and Phthalazin-thione Systems作者:Eisuke Sato、Yoshiya Ikeda、Yuichi KanaokaDOI:10.1246/cl.1987.273日期:1987.2.5Irradiation of quinolin-2-, isoquinolin-1-, and phthalazin-1-thiones with olefins afforded photoaddition products: substituted quinolines, isoquinolines and phthalazines at the thiocarbonyl carbon. A new addition-cyclization was found producing tricyclic thiopyrano-isoquinolines and -phthalazines.
-
Photochemistry of conjugated nitrogen-thiocarbonyl systems. VII. Photoaddition of quinoline-, isoquinoline- and phthalazine-thione systems to olefins.作者:Eisuke SATO、Yoshiya IKEDA、Yuichi KANAOKADOI:10.1248/cpb.38.1205日期:——Photolyses of quinoline-2-thiones (1A, B), isoquinoline-1-thione (14A), and phthalazine-1-thiones (14B-D) in the presence of olefins (2) afforded photoaddition products such as substituted quinolines, isoquinolines, phthalazines, and novel tricyclic products, probably by way of thietanes formed by photoaddition of olefins to the C=S bond.
-
Phtalazin derivatives and their use as pesticides
-
Carbenoid intermediates in the synthesis of mesoionic anhydro-4-hydroxythiazolium hydroxides作者:Kevin T. Potts、Peter MurphyDOI:10.1039/c39840001348日期:——α-(Tosylhydrazono)phenylacetyl chloride and N-Monosubstituted thioamides in dry, non-protic solvents and in the presence of Et3N give representatives of the title mesoionic ring system in good to excellent yields; carbene or carbenoid type intermediates are most likely involved in these regiospecific cyclizations.
表征谱图
-
氢谱1HNMR
-
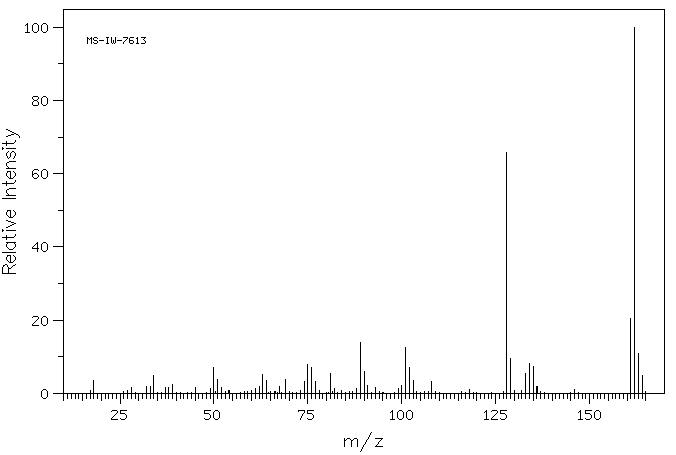
质谱MS
-
碳谱13CNMR
-
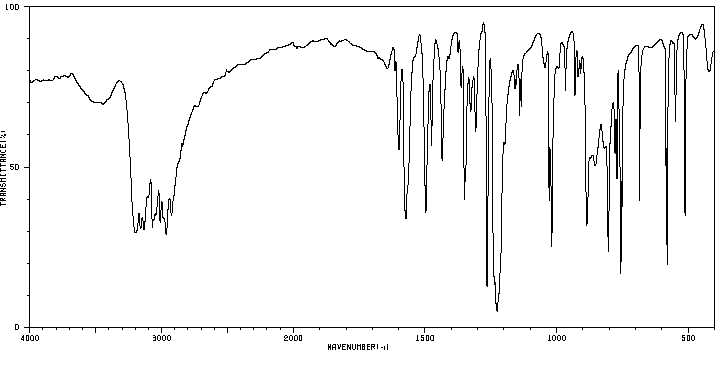
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮