氨基胍媒染剂 | 79-17-4
中文名称
氨基胍媒染剂
中文别名
氨基胍;匹马吉定;脒基联氨
英文名称
aminoguanidine
英文别名
2-aminoguanidine
CAS
79-17-4
化学式
CH6N4
mdl
MFCD00242587
分子量
74.0854
InChiKey
HAMNKKUPIHEESI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:182-185 °C (decomp)
-
沸点:159.5±23.0 °C(Predicted)
-
密度:1.72±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-1.5
-
重原子数:5
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:90.4
-
氢给体数:3
-
氢受体数:2
安全信息
-
储存条件:库房应保持通风、低温和干燥的环境,并将物品与氧化剂和酸类分开存放。
SDS
制备方法与用途
上下游信息
反应信息
-
作为反应物:参考文献:名称:一种合成5-氨基四唑的方法摘要:一种合成5‑氨基四唑的方法,以水合肼、石灰氮、亚硝酸钠、无机酸和无机碱为原料合成得到5‑氨基四唑。本方法通过复分解反应、加成反应和重氮异构化反应将石灰氮的氰基率先转化为氨基胍,然后发生氨基胍异构化反应合成5‑氨基四唑。本发明与传统方法相比,具有以下优点:(1)以价格低廉的原料为起始原材料,合成得到产品;(2)简化5‑氨基四唑的操作工艺,减少反应溶剂种类和工艺操作的复杂性,降低了原料成本和生产成本,降低了5‑氨基四唑的合成成本,提高了产品的市场竞争力。具有效率高、收率高、成本低、易操作等优点。公开号:CN109761925A
-
作为产物:参考文献:名称:Reduction of Nitroguanidine. IX. The Reduction of Nitrosoguanidine to Aminoguanidine1摘要:DOI:10.1021/ja01289a014
-
作为试剂:描述:、 1-氨基-11-叠氮-3,6,9-三氧杂十一烷 在 copper(II) sulfate 、 sodium ascorbate 、 氨基胍媒染剂 、 三(3-羟丙基三唑甲基)胺 作用下, 以 水 、 叔丁醇 为溶剂, 反应 1.0h, 以0.27 mg的产率得到参考文献:名称:甲硅烷基保护的炔丙基甘氨酸,用于通过化学选择性甲硅烷基脱保护对肽进行多重标记摘要:我们合成了带有不同甲硅烷基保护基团的 Fmoc-炔丙基甘氨酸衍生物,可以使用标准固相肽偶联程序轻松引入这些基团。利用不同甲硅烷基保护基团之间的正交性,证明了通过点击反应将功能分子化学选择性地结合到 19 聚体肽中。DOI:10.1016/j.tetlet.2021.153093
文献信息
-
Discovery of Phthalimides as Immunomodulatory and Antitumor Drug Prototypes作者:Claudia Pessoa、Paulo Michel P. Ferreira、Letícia Veras C. Lotufo、Manoel O. de Moraes、Suellen M. T. Cavalcanti、Lucas Cunha D. Coêlho、Marcelo Z. Hernandes、Ana Cristina L. Leite、Carlos A. De Simone、Vlaudia M. A. Costa、Valdênia M. O. SouzaDOI:10.1002/cmdc.200900525日期:2010.4.6Optimized anticancer agents: A set of functionalized phthalimides was synthesized and evaluated for antitumor activities and as modulators of the secretion of cytokines and nitric oxide. Their potency was compared with that of thalidomide, and as a result, a new potent anticancer and immunomodulatory agent, compound 2 b, was identified.
-
[EN] TRIAZOLOTRIAZINE DERIVATIVES AS A2A RECEPTOR ANTAGONISTS<br/>[FR] DÉRIVÉS DE TRIAZOLOTRIAZINE EN TANT QU'ANTAGONISTES DU RÉCEPTEUR A2A申请人:ZHEJIANG VIMGREEN PHARMACEUTICALS LTD公开号:WO2020002969A1公开(公告)日:2020-01-02The present invention provides triazolotriazine derivatives of formula (1) as A2A receptor antagonists. Compounds of formula (1) and pharmaceutical compositions including the compounds can be used for the treatment of disorders related to A2A receptor hyperfunctioning, such as certain types cancers. Compounds of formula (1) and methods of preparing the compounds are disclosed in the invention.本发明提供了公式(1)的三唑三嗪衍生物作为A2A受体拮抗剂。公式(1)的化合物和包括这些化合物的药物组合物可用于治疗与A2A受体过度功能有关的疾病,如某些类型的癌症。该发明揭示了公式(1)的化合物和制备这些化合物的方法。
-
Microscale Parallel Synthesis of Acylated Aminotriazoles Enabling the Development of Factor XIIa and Thrombin Inhibitors作者:Simon Platte、Marvin Korff、Lukas Imberg、Ilker Balicioglu、Catharina Erbacher、Jonas M. Will、Constantin G. Daniliuc、Uwe Karst、Dmitrii V. KalininDOI:10.1002/cmdc.202100431日期:2021.12.14approach toward N-acylated aminotriazoles is reported, enabling the compounds’ screening against FXIIa and thrombin. This approach afforded low-nanomolar FXIIa and thrombin inhibitors with no off-targeting of the other tested serine proteases. Selected compounds were shown to be covalent inhibitors of FXIIa and demonstrated anticoagulant properties in vitro, influencing the intrinsic blood coagulation
-
Fenbufen, a New Anti-Inflammatory Analgesic: Synthesis and Structure-Activity Relationships of Analogs作者:Ralph G. Child、Arnold C. Osterberg、Adolph E. Sloboda、Andrew S. TomcufcikDOI:10.1002/jps.2600660403日期:1977.4hundred analogs of fenbufen were prepared and tested using the carrageenan, polyarthritis, and UV erythema anti-inflammatory tests and the 2-phenyl-1,4-benzoquinone writhing and inflamed paw pressure analgesic tests. Only three retained the same full spectrum of activity as fenbufen: dl-4-(4-biphenylyl)-4-hydroxybutyric acid, dl-4-(4-biphenylyl)-1,4-butanediol, and 4-biphenylacetic acid. Fenbufen had the
-
Effective Synthesis and Cytotoxic Activity of Methyl Maleopimarate Imides作者:Ilshat Maratovich Sakhautdinov、Rauilya Nadirovna Malikova、Diana Valievna Khasanova、Liana Fanzilevna Zainullina、Vener Absatarovich Vakhitov、Alexander Nikolaevich Lobov、Yuliya Venerovna Vakhitova、Marat Sabirovich YunusovDOI:10.2174/1570178615666180212154722日期:2018.8.8two-fold excess of amines and ultrasonic influence increased yield of target products and reduced reaction time. The structures of the products were proved by two-dimensional correlation spectra of HSQC, HMBC, COSY, NOESY. A new efficient method for the synthesis of a large group of hybrid potentially biologically active compounds by condensation of methyl maleopimarate with various amines (glycine, β-alanine现代生物化学的一个持续存在的问题是寻找基于具有各种抗癌活性并且同时对整个生物体正常细胞影响很小的具有不同药效基团的天然加合物合成新杂合化合物的方法。 在DMSO中用不同的胺处理从松香中得到的马来酸甲酯MMP,通过60分钟的热缩合和30分钟的超声(US)处理缩合得到马来酸酰亚胺。所有合成的化合物(包括母体化合物-马来酸和MMP)都在HEK293(人类胚胎肾细胞)和Jurkat细胞(人类T细胞淋巴母细胞样细胞系)中进行了体外细胞毒性MTT分析。通过开发的方法,获得了一系列新的马来酰亚胺。发现使用两倍过量的胺和超声影响增加了目标产物的产率并缩短了反应时间。通过HSQC,HMBC,COSY,NOESY的二维相关光谱证明了产物的结构。 一种新的有效方法,可通过马来酸马来酸甲酯与各种胺(甘氨酸,β-丙氨酸,γ-氨基丁酸,氨基己酸,α-丙氨酸,β-苯基-α-开发了在二甲基亚砜(DMSO)中超声作用下的丙
表征谱图
-
氢谱1HNMR
-
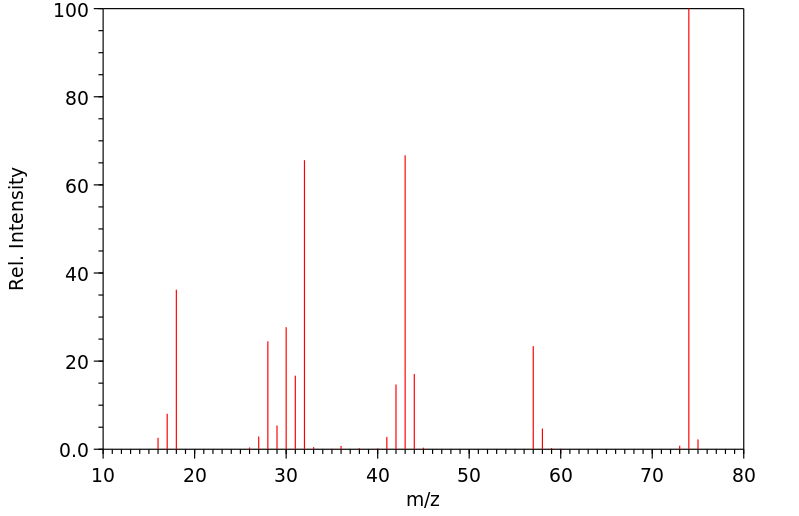
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷