2,4,4,6-四甲基-1-氧杂-3-氮杂-2-环己烯 | 26939-18-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.875
-
拓扑面积:21.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险等级:3
-
安全说明:S24/25
-
海关编码:2934999090
-
包装等级:III
-
危险品运输编号:UN 1993
-
储存条件:1. 保持贮藏器密封。 2. 将其存放在紧密的容器中,并储存在阴凉、干燥的地方。
SDS
| Name: | 5 6-Dihydro-2 4 4 6-Tetramethyl-4H-1 3-Oxazine Tech. 85% Material Safety Data Sheet |
| Synonym: | 4H-1,3-Oxazine, 5,6-Dihydro-2,4,4,6-Tetramethyl |
| CAS: | 26939-18-4 |
Synonym:4H-1,3-Oxazine, 5,6-Dihydro-2,4,4,6-Tetramethyl
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 26939-18-4 | 4H-1,3-Oxazine, 5,6-Dihydro-2,4,4,6-Te | 85 | 248-117-8 |
Risk Phrases: 10
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable.The toxicological properties of this material have not been fully investigated.Hygroscopic (absorbs moisture from the air).
Potential Health Effects
Eye:
May cause eye irritation. May cause chemical conjunctivitis and corneal damage.
Skin:
May cause irritation and dermatitis. May cause cyanosis of the extremities.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated. Ingestion of large amounts may cause CNS depression.
Inhalation:
The toxicological properties of this substance have not been fully investigated. Aspiration may lead to pulmonary edema. Vapors may cause dizziness or suffocation. May cause burning sensation in the chest.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air.
Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Will burn if involved in a fire. Use water spray to keep fire-exposed containers cool. Water may be ineffective. Material is lighter than water and a fire may be spread by the use of water. Containers may explode in the heat of a fire.
Flammable liquid and vapor. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam. For large fires, use water spray, fog, or alcohol-resistant foam. Use water spray to cool fire-exposed containers. Water may be ineffective. Do NOT use straight streams of water.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation. A vapor suppressing foam may be used to reduce vapors.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Flammables-area. Store protected from moisture.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 26939-18-4: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear orange-red
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 47 - 49 deg C @ 17.00mm Hg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 45 deg C ( 113.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .8860g/cm3
Molecular Formula: C8H15NO
Molecular Weight: 141.21
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat, strong oxidants, exposure to moist air or water.
Incompatibilities with Other Materials:
Moisture, strong acids, strong bases, strong oxidizing agents, strong reducing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 26939-18-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
4H-1,3-Oxazine, 5,6-Dihydro-2,4,4,6-Tetramethyl - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
R 10 Flammable.
Safety Phrases:
S 9 Keep container in a well-ventilated place.
S 16 Keep away from sources of ignition - No
smoking.
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 33 Take precautionary measures against static
discharges.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 26939-18-4: No information available.
Canada
CAS# 26939-18-4 is listed on Canada's NDSL List.
CAS# 26939-18-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 26939-18-4 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
在装有温度计、搅拌器和250毫升滴液漏斗的2升烧瓶中,加入400毫升浓硫酸(95-97%)。将烧瓶置于冰浴中冷却至0-5℃并保持此温度。在此条件下,逐滴滴加90.2克(2.2摩尔)乙腊,滴完后继续在相同低温条件下滴加236克(2.0摩尔)2-甲基-2,4-戊二醇。将反应混合物搅拌1小时后,将其倾倒在大约1500克碎冰上。水溶液用四份每份125毫升的氯仿萃取,弃去氯仿萃取液。
然后向水溶液中加入40%氢氧化钠溶液,使其呈碱性,在此过程中不断加入碎冰以保持混合物温度低于35℃。当溶液呈碱性后,分离出黄色油状物。水层用三份每份100毫升的乙醚萃取,合并乙醚萃取液和油状物,并用无水碳酸钾干燥。使用旋转蒸发器蒸去乙醚,残余物通过长25厘米的分馏柱蒸馏,最终得到183.2克(65%)的2,4,4,6-四甲基二氢-1,3-恶唑。蒸馏时产物会严重起泡,使用玻璃毛蒸馏可以避免此现象。
合成制备方法在装有温度计、搅拌器和250毫升滴液漏斗的2升烧瓶中,加入400毫升浓硫酸(95-97%)。将烧瓶置于冰浴中冷却至0-5℃并保持此温度。在此条件下,逐滴滴加90.2克(2.2摩尔)乙腊,滴完后继续在相同低温条件下滴加236克(2.0摩尔)2-甲基-2,4-戊二醇。将反应混合物搅拌1小时后,将其倾倒在大约1500克碎冰上。水溶液用四份每份125毫升的氯仿萃取,弃去氯仿萃取液。
然后向水溶液中加入40%氢氧化钠溶液,使其呈碱性,在此过程中不断加入碎冰以保持混合物温度低于35℃。当溶液呈碱性后,分离出黄色油状物。水层用三份每份100毫升的乙醚萃取,合并乙醚萃取液和油状物,并用无水碳酸钾干燥。使用旋转蒸发器蒸去乙醚,残余物通过长25厘米的分馏柱蒸馏,最终得到183.2克(65%)的2,4,4,6-四甲基二氢-1,3-恶唑。蒸馏时产物会严重起泡,使用玻璃毛蒸馏可以避免此现象。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-hydroxymethyl-4,4,6-trimethyl-5,6-dihydro-(4H)-1,3-oxazine 86354-09-8 C8H15NO2 157.213 —— 3-(4,4,6-Trimethyl-5,6-dihydro-4H-1,3-oxazinyl-(2))-propanol-(1) 22944-86-1 C10H19NO2 185.266 —— 2-(3-Ethoxy-propyl)-4,4,6-trimethyl-5,6-dihydro-4H-1,3-oxazin 40228-05-5 C12H23NO2 213.32 —— 2-(5-chloro-pentyl)-4,4,6-trimethyl-5,6-dihydro-4H-[1,3]oxazine 36871-49-5 C12H22ClNO 231.766 —— 4,4,6-trimethyl-2-(trimethylsilanyl-methyl)-5,6-dihydro-4H-[1,3]oxazine 50289-70-8 C11H23NOSi 213.395 —— 2-Cyclopentyl-4,4,6-trimethyl-5,6-dihydro-1,3-oxazin 39576-00-6 C12H21NO 195.305 —— 5,6-dihydro-4,4,6-trimethyl-2-[2-(4-fluorophenyl)ethyl]-4H-1,3-oxazine 63416-68-2 C15H20FNO 249.328 —— 2-(bis-trimethylsilanyl-methyl)-4,4,6-trimethyl-5,6-dihydro-4H-[1,3]oxazine 62833-39-0 C14H31NOSi2 285.577
反应信息
-
作为反应物:参考文献:名称:2-硫代甲硅烷基甲基-1,3-恶嗪与羰基化合物的反应。立体选择性烯烃合成的便捷途径摘要:DOI:10.1016/s0040-4039(00)92570-x
-
作为产物:描述:参考文献:名称:Kuznetsov; Brusilovskii, Yu E., Russian Journal of Organic Chemistry, 2000, vol. 36, # 1, p. 138 - 139摘要:DOI:
文献信息
-
Synthesis, activation, and cytotoxicity of aldophosphamide analogs作者:Richard F. Borch、Ronald R. ValenteDOI:10.1021/jm00114a014日期:1991.10tautomerization to an enamine intermediate might provide a mechanistic alternative to the beta-elimination reaction for release of phosphoramide mustard. The 4,4,6-trimethyltetrahydro-1,3-oxazine moiety was selected on the basis of its rapid rate of iminium ion generation and relatively slow rate of hydrolysis. These analogues underwent phosphorodiamidate release by three distinct mechanisms: hydrolysis to aldophosphamide制备了一系列醛磷酰胺的全氢恶嗪类似物,并对其31P NMR动力学和体外细胞毒性进行了评估。这些化合物是基于这样的想法而开发的,即开环和互变异构化为烯胺中间体可能为释放磷酰胺芥菜的β-消除反应提供一种机械替代方法。选择4,4,6-三甲基四氢-1,3-恶嗪部分是基于其亚胺离子生成速度快和水解速度相对慢的原因。这些类似物通过三种不同的机制释放二氨基磷酸酯:水解为醛基磷酸酰胺和随后的β-消除;环化生成4-羟基环磷酰胺,该环磷酰胺通过开环和消除作用释放二氨基磷酸酯;并通过快速排出二氨基氨基磷酸酯而互变为烯胺。动力学研究表明,水解成醛对整个活化过程的贡献最小,烯胺途径代表了活化的主要途径。对于那些可能经历环化的类似物,该途径与烯胺释放有效竞争,并且这些类似物在细胞毒性上基本上等同于它们的4-羟基环磷酰胺。制备了一系列不能进行环化的四-N-取代的磷酸二氨基甲酸酯,以探索环化对这些类似物的细胞毒性的影响。
-
Phosphoramidate analogs of 2'-deoxyuridine申请人:University of Rochester公开号:US05233031A1公开(公告)日:1993-08-03The present invention provides a series of cytotoxic phosphoramidate analogs of 5-fluoro-2'-deoxyuridine of the general formula (I): ##STR1## wherein R.sup.1 is H, F or (C.sub.1 -C.sub.4)alkyl; R.sup.2 is CH.sub.2 CH.sub.2 X wherein X is Cl, Br, I or p-toluenesulfonyl; R.sup.3 is (C.sub.1 -C.sub.4)alkyl or CH.sub.2 CH.sub.2 X wherein X is Cl, Br, I or p-toluenesulfonyl; or wherein R.sup.2 and R.sup.3, taken together with the N atom, can be a 5- or 6-membered heterocyclic ring which is aliphatic or aliphatic interrupted by a ring oxygen or a second ring nitrogen; R.sup.4 is H, one equivalent of a pharmaceutically-acceptable cation or (4,4,6-trimethyltetrahydro-1,3-oxazin-2-yl)ethyl, and the pharmaceutically-acceptable salts thereof.本发明提供了一系列细胞毒性的5-氟-2'-脱氧尿嘧啶的磷酰胺类似物,其一般式为(I):##STR1##其中R.sup.1为H,F或(C.sub.1 -C.sub.4)烷基;R.sup.2为CH.sub.2 CH.sub.2 X,其中X为Cl,Br,I或对甲苯磺酰基;R.sup.3为(C.sub.1 -C.sub.4)烷基或CH.sub.2 CH.sub.2 X,其中X为Cl,Br,I或对甲苯磺酰基;或其中R.sup.2和R.sup.3,与N原子一起,可以是一个5-或6-成员杂环,其为脂肪族的或被一个环氧原子或第二个环氮原子中断的脂肪族;R.sup.4为H,一个等效的药用阳离子或(4,4,6-三甲基四氢-1,3-噁唑啉-2-基)乙基,以及其药用盐。
-
[EN] PYRANO-QUINOLINES, PYRANO-QUINOLINONES, COMBINATIONS THEREOF, PHOTOCHROMIC COMPOSITIONS AND ARTICLES<br/>[FR] PYRANO-QUINOLEINES, PYRANO-QUINOLINONES, LEURS COMBINAISONS, ET COMPOSITIONS ET ARTICLES PHOTOCHROMES申请人:TRANSITIONS OPTICAL INC公开号:WO2005061514A1公开(公告)日:2005-07-07Described are compositions of at least one material represented by a pyrano[3,2-c]quinoline structure, a pyrano[3,2-c]quinolinone structure or mixtures thereof. The pyrano[3,2-c]quinoline structure is characterized by having a nitrogen atom at the 6-position ring atom and an oxy-substituent at the 5-position ring atom. The pyrano[3,2-c]quinolinone structure is characterized by having a substituted nitrogen atom at the 6-position ring atom and an oxo-substituent at the 5-position ring atom, the nitrogen atom substituents being hydrogen, aliphatic substituents, cycloaliphatic substituents, aromatic substituents, heteroaromatic substituents or a combination thereof. Both of the pyrano[3,2-c]quinoline and pyrano[3,2-c]quinolinone structures are characterized by having two substituents at the 2-position ring atom, each substituent being independently chosen from aliphatic substituents, cycloaliphatic substituents, aromatic substituents, heteroaromatic substituents or a combination thereof, provided that both substituents at the 2-position are not aliphatic. Alternatively, the two substituents at the 2-position can combine to form a spirocyclic group, provided that the spirocyclic group is not norbornylidene or bicyclo[3.3.1]9-nonylidene. The ring atoms have been numbered according to the International Union of Pure and Applied Chemistry rules of nomenclature starting with the 1-position ring atom being the oxygen atom of the pyran ring and numbering counterclockwise therefrom. Also described are photochromic articles that contain or that have coatings or films containing at least one of the novel compositions or combinations thereof with other photochromic materials.描述了由吡喃并[3,2-c]喹啉结构、吡喃并[3,2-c]喹啉酮结构或它们的混合物表示的至少一种材料的组合物。吡喃并[3,2-c]喹啉结构的特征是在6位环原子处有一个氮原子和在5位环原子处有一个氧基取代物。吡喃并[3,2-c]喹啉酮结构的特征是在6位环原子处有一个取代的氮原子和在5位环原子处有一个氧基取代物,氮原子的取代物是氢、脂肪取代物、环脂取代物、芳香取代物、杂芳取代物或它们的组合。吡喃并[3,2-c]喹啉和吡喃并[3,2-c]喹啉酮结构的特征是在2位环原子处有两个取代基,每个取代基独立地选择自脂肪取代物、环脂取代物、芳香取代物、杂芳取代物或它们的组合,前提是2位处的两个取代基不是脂肪取代物。或者,2位处的两个取代基可以结合形成一个螺环烷基团,前提是螺环烷基团不是去甲基环戊烯基或双环[3.3.1]9-壬烯基。根据国际纯粹与应用化学联合会的命名规则,环原子已经编号,从吡喃环的氧原子作为1位环原子开始,逆时针编号。还描述了包含或含有至少一种新型组合物或与其他光致变色材料的组合物的光致变色物品的涂层或薄膜。
-
Radical addition-coupling polymerization with various nitroso compounds作者:Junjie Li、Qi WangDOI:10.1002/pola.27061日期:2014.3.15Four nitroso esters were prepared by oxidation of 4,4‐dimethyl dihydro‐1,3‐oxazine or 4,4‐dimethyl‐2‐oxazoline with two equiv of m‐chloroperoxybenzoic acid. All of them can be applied in radical addition‐coupling polymerization to produce periodic polymer together with introduction of ester group at side chain. Compared with 2‐methyl‐2‐nitrosopropane, 2‐nitroso‐2‐methyl‐4‐acetoxypentane and 2‐methyl‐2‐nitrosopropyl
-
An efficient total synthesis of propylure, the highly active sex attractant for the pink bollworm moth作者:A.I. Meyers、E.W. CollingtonDOI:10.1016/s0040-4020(01)91762-4日期:1971.1The total synthesis of trans-1-acetoxy-10-(n-propyl)trideca-5,9-diene (propylure), a substance which induces highly active sexual response in the male bollworm moth has been accomplished using several novel synthetic methods. The efficiency of the techniques employed are demonstrated by an overall yield of 31 % for the sex attractant.
表征谱图
-
氢谱1HNMR
-
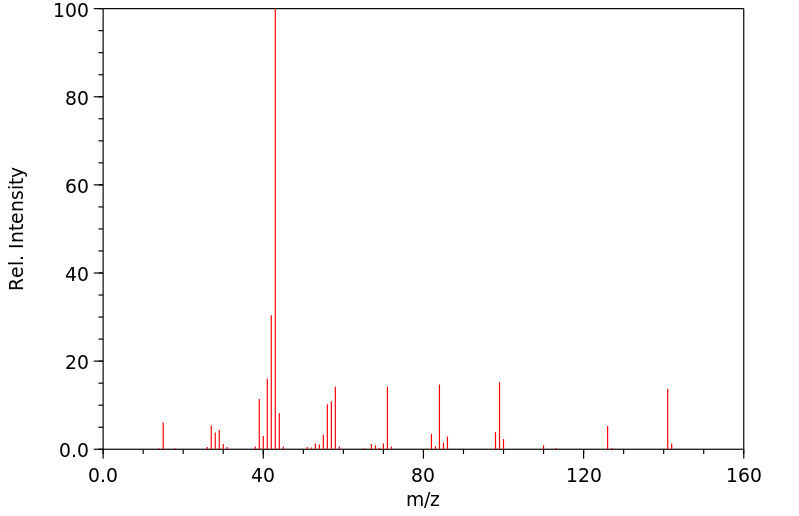
质谱MS
-
碳谱13CNMR
-
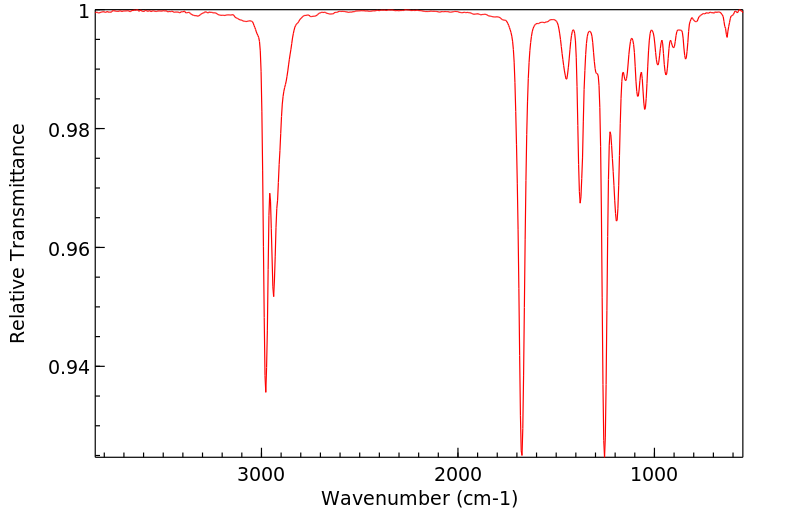
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息